A thermophilic cell-free cascade enzymatic reaction for acetoin synthesis from pyruvate
- PMID: 28659601
- PMCID: PMC5489476
- DOI: 10.1038/s41598-017-04684-8
A thermophilic cell-free cascade enzymatic reaction for acetoin synthesis from pyruvate
Abstract
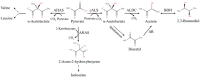
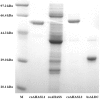
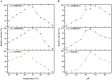
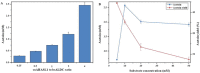
Acetoin (3-hydroxy-2-butanone) is an important bio-based platform chemical with wide applications. In vitro enzyme catalysed synthesis exhibits great feasibility in the production of chemicals with high purity. In the present work, a synthetic pathway involving a two-step continuous reaction was constructed in vitro for acetoin production from pyruvate at improved temperature. Thermostable candidates, acetolactate synthase (coAHASL1 and coAHASL2 from Caldicellulosiruptor owensensis OL) and α-acetolactate decarboxylase (bsALDC from Bacillus subtilis IPE5-4) were cloned, heterologously expressed, and characterized. All the enzymes showed maximum activities at 65-70 °C and pH of 6.5. Enzyme kinetics analysis showed that coAHASL1 had a higher activity but lower affinity against pyruvate than that of coAHASL2. In addition, the activities of coAHASL1 and bsALDC were promoted by Mn2+ and NADPH. The cascade enzymatic reaction was optimized by using coAHASL1 and bsALDC based on their kinetic properties. Under optimal conditions, a maximum concentration of 3.36 ± 0.26 mM acetoin was produced from 10 mM pyruvate after reaction for 24 h at 65 °C. The productivity of acetoin was 0.14 mM h-1, and the yield was 67.80% compared with the theoretical value. The results confirmed the feasibility of synthesis of acetoin from pyruvate with a cell-free enzyme catalysed system at improved temperature.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- Zhu CJ, et al. Production of liquid hydrocarbon fuels with acetoin and platform molecules derived from lignocellulose. Green Chem. 2016;18:2165–2174. doi: 10.1039/C5GC02414E. - DOI
-
- Werpy, T. & Petersen, G. Top value added chemicals from biomass: Volume I - Results of screening for potential candidates from sugars and synthesis gas. Report No. DOE/GO-102004-1992; TRN: US200427%%671, doi:10.2172/15008859, Medium: ED; Size: 76 pp. pages (2004).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

