A novel humanized mouse lacking murine P450 oxidoreductase for studying human drug metabolism
- PMID: 28659616
- PMCID: PMC5489481
- DOI: 10.1038/s41467-017-00049-x
A novel humanized mouse lacking murine P450 oxidoreductase for studying human drug metabolism
Erratum in
-
Erratum: A novel humanized mouse lacking murine p450 oxidoreductase for studying human drug metabolism.Nat Commun. 2017 Oct 17;8(1):984. doi: 10.1038/s41467-017-01314-9. Nat Commun. 2017. PMID: 29042563 Free PMC article.
Abstract
Only one out of 10 drugs in development passes clinical trials. Many fail because experimental animal models poorly predict human xenobiotic metabolism. Human liver chimeric mice are a step forward in this regard, as the human hepatocytes in chimeric livers generate human metabolites, but the remaining murine hepatocytes contain an expanded set of P450 cytochromes that form the major class of drug-metabolizing enzymes. We therefore generated a conditional knock-out of the NADPH-P450 oxidoreductase (Por) gene combined with Il2rg - /- /Rag2 - /- /Fah - /- (PIRF) mice. Here we show that homozygous PIRF mouse livers are readily repopulated with human hepatocytes, and when the murine Por gene is deleted (<5%), they predominantly use human cytochrome metabolism. When given the anticancer drug gefitinib or the retroviral drug atazanavir, the Por-deleted humanized PIRF mice develop higher levels of the major human metabolites than current models. Humanized, murine Por-deficient PIRF mice can thus predict human drug metabolism and should be useful for preclinical drug development.Human liver chimeric mice are increasingly used for drug testing in preclinical development, but express residual murine p450 cytochromes. Here the authors generate mice lacking the Por gene in the liver, and show that human cytochrome metabolism is used following repopulation with human hepatocytes.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
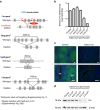
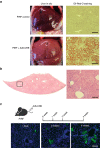
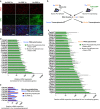
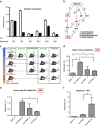
References
-
- Nelson DR, Zeldin DC, Hoffman SM, Maltais LJ, Wain HM, Nebert DW. Comparison of cytochrome P450 (CYP) genes from the mouse and human genomes, including nomenclature recommendations for genes, pseudogenes and alternative-splice variants. Pharmacogenetics. 2004;14:1–18. doi: 10.1097/00008571-200401000-00001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

