Expression, Localization of SUMO-1, and Analyses of Potential SUMOylated Proteins in Bubalus bubalis Spermatozoa
- PMID: 28659810
- PMCID: PMC5468435
- DOI: 10.3389/fphys.2017.00354
Expression, Localization of SUMO-1, and Analyses of Potential SUMOylated Proteins in Bubalus bubalis Spermatozoa
Abstract
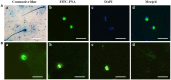
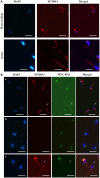
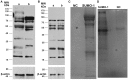
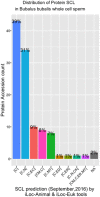
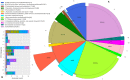
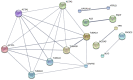
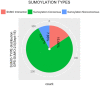
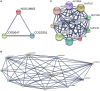
Mature spermatozoa have highly condensed DNA that is essentially silent both transcriptionally and translationally. Therefore, post translational modifications are very important for regulating sperm motility, morphology, and for male fertility in general. Protein sumoylation was recently demonstrated in human and rodent spermatozoa, with potential consequences for sperm motility and DNA integrity. We examined the expression and localization of small ubiquitin-related modifier-1 (SUMO-1) in the sperm of water buffalo (Bubalus bubalis) using immunofluorescence analysis. We confirmed the expression of SUMO-1 in the acrosome. We further found that SUMO-1 was lost if the acrosome reaction was induced by calcium ionophore A23187. Proteins modified or conjugated by SUMO-1 in water buffalo sperm were pulled down and analyzed by mass spectrometry. Sixty proteins were identified, including proteins important for sperm morphology and motility, such as relaxin receptors and cytoskeletal proteins, including tubulin chains, actins, and dyneins. Forty-six proteins were predicted as potential sumoylation targets. The expression of SUMO-1 in the acrosome region of water buffalo sperm and the identification of potentially SUMOylated proteins important for sperm function implicates sumoylation as a crucial PTM related to sperm function.
Keywords: Bubalus bubalis; SUMO-1; post translational modification; protein; spermatozoa.
Figures


Similar articles
-
Localization and identification of sumoylated proteins in human sperm: excessive sumoylation is a marker of defective spermatozoa.Hum Reprod. 2013 Jan;28(1):210-23. doi: 10.1093/humrep/des317. Epub 2012 Oct 17. Hum Reprod. 2013. PMID: 23077236 Free PMC article.
-
SUMO1 in human sperm: new targets, role in motility and morphology and relationship with DNA damage.Reproduction. 2014 Nov;148(5):453-67. doi: 10.1530/REP-14-0173. Epub 2014 Aug 12. Reproduction. 2014. PMID: 25118297
-
Supplementation of l-tryptophan (an aromatic amino acid) in tris citric acid extender enhances post-thaw progressive motility, plasmalemma, mitochondrial membrane potential, acrosome, and DNA integrities, and in vivo fertility rate of buffalo (Bubalus bubalis) bull spermatozoa.Cryobiology. 2020 Feb 1;92:117-123. doi: 10.1016/j.cryobiol.2019.11.044. Epub 2019 Nov 26. Cryobiology. 2020. PMID: 31783000
-
Posttranslational Modifications in Spermatozoa and Effects on Male Fertility and Sperm Viability.OMICS. 2017 May;21(5):245-256. doi: 10.1089/omi.2016.0173. OMICS. 2017. PMID: 28481731 Review.
-
Analysis of Protein Sumoylation.Curr Protoc Protein Sci. 2016 Feb 2;83:14.8.1-14.8.8. doi: 10.1002/0471140864.ps1408s83. Curr Protoc Protein Sci. 2016. PMID: 26836406 Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

