Cobalt Chloride Upregulates Impaired HIF-1α Expression to Restore Sevoflurane Post-conditioning-Dependent Myocardial Protection in Diabetic Rats
- PMID: 28659817
- PMCID: PMC5468378
- DOI: 10.3389/fphys.2017.00395
Cobalt Chloride Upregulates Impaired HIF-1α Expression to Restore Sevoflurane Post-conditioning-Dependent Myocardial Protection in Diabetic Rats
Abstract
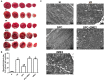
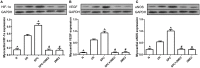
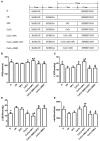
Previous studies from our group have demonstrated that sevoflurane post-conditioning (SPC) protects against myocardial ischemia reperfusion injury via elevating the intranuclear expression of hypoxia inducible factor-1 alpha (HIF-1α). However, diabetic SPC is associated with decreased myocardial protection and disruption of the HIF-1 signaling pathway. Previous studies have demonstrated that cobalt chloride (CoCl2) can upregulate HIF-1α expression under diabetic conditions, but whether myocardial protection by SPC can be restored afterward remains unclear. We established a rat model of type 2 diabetes and a Langendorff isolated heart model of ischemia-reperfusion injury. Prior to reperfusion, 2.4% sevoflurane was used as a post-conditioning treatment. The diabetic rats were treated with CoCl2 24 h before the experiment. At the end of reperfusion, tests were performed to assess myocardial function, infarct size, mitochondrial morphology, nitric oxide (NO), Mitochondrial reactive oxygen species (ROS), mitochondrial respiratory function and enzyme activity, HIF-1α, vascular endothelial growth factor (VEGF) and endothelial NO synthase (eNOS) protein levels. In addition, myocardial protection by SPC was monitored after the blood glucose levels were lowered by insulin. The diabetic state was associated with deficient SPC protection and decreased HIF-1α expression. After treating the diabetic rats with CoCl2, SPC significantly upregulated the expression of HIF-1α, VEGF and eNOS, which markedly improved cardiac function, NO, mitochondrial respiratory function, and enzyme activity and decreased the infarction areas and ROS. In addition, these effects were not influenced by blood glucose levels. This study proved that CoCl2activates the HIF-1α signaling pathway, which restores SPC-dependent myocardial protection under diabetic conditions, and the protective effects of SPC were independent of blood glucose levels.
Keywords: diabetic state; hypoxia inducible factor-1; ischemia-reperfusion; myocardial protection; sevoflurane post-conditioning.
Figures

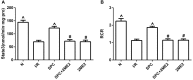


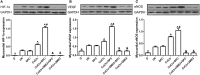
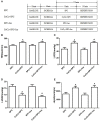

Comment in
-
Commentary: Neutral Commentary on Frontiers Article "Cobalt Chloride Upregulates Impaired HIF-1α Expression to Restore Sevoflurane Post-conditioning-Dependent Myocardial Protection in Diabetic Rats".Front Physiol. 2017 Nov 21;8:926. doi: 10.3389/fphys.2017.00926. eCollection 2017. Front Physiol. 2017. PMID: 29209228 Free PMC article. No abstract available.
References
-
- Akinrinde A. S., Omobowale O., Oyagbemi A., Asenuga E., Ajibade T. (2016). Protective effects of kolaviron and gallic acid against cobalt-chloride-induced cardiorenal dysfunction via suppression of oxidative stress and activation of the ERK signaling pathway. Can. J. Physiol. Pharmacol. 94, 1276–1284. 10.1139/cjpp-2016-0197 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

