Brain Activation by H1 Antihistamines Challenges Conventional View of Their Mechanism of Action in Motion Sickness: A Behavioral, c-Fos and Physiological Study in Suncus murinus (House Musk Shrew)
- PMID: 28659825
- PMCID: PMC5470052
- DOI: 10.3389/fphys.2017.00412
Brain Activation by H1 Antihistamines Challenges Conventional View of Their Mechanism of Action in Motion Sickness: A Behavioral, c-Fos and Physiological Study in Suncus murinus (House Musk Shrew)
Abstract
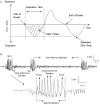
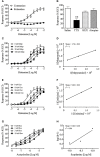
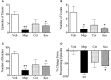
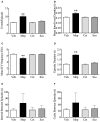
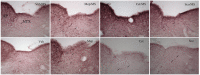
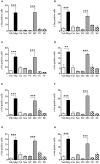
Motion sickness occurs under a variety of circumstances and is common in the general population. It is usually associated with changes in gastric motility, and hypothermia, which are argued to be surrogate markers for nausea; there are also reports that respiratory function is affected. As laboratory rodents are incapable of vomiting, Suncus murinus was used to model motion sickness and to investigate changes in gastric myoelectric activity (GMA) and temperature homeostasis using radiotelemetry, whilst also simultaneously investigating changes in respiratory function using whole body plethysmography. The anti-emetic potential of the highly selective histamine H1 receptor antagonists, mepyramine (brain penetrant), and cetirizine (non-brain penetrant), along with the muscarinic receptor antagonist, scopolamine, were investigated in the present study. On isolated ileal segments from Suncus murinus, both mepyramine and cetirizine non-competitively antagonized the contractile action of histamine with pK b values of 7.5 and 8.4, respectively; scopolamine competitively antagonized the contractile action of acetylcholine with pA2 of 9.5. In responding animals, motion (1 Hz, 4 cm horizontal displacement, 10 min) increased the percentage of the power of bradygastria, and decreased the percentage power of normogastria whilst also causing hypothermia. Animals also exhibited an increase in respiratory rate and a reduction in tidal volume. Mepyramine (50 mg/kg, i.p.) and scopolamine (10 mg/kg, i.p.), but not cetirizine (10 mg/kg, i.p.), significantly antagonized motion-induced emesis but did not reverse the motion-induced disruptions of GMA, or hypothermia, or effects on respiration. Burst analysis of plethysmographic-derived waveforms showed mepyramine also had increased the inter-retch+vomit frequency, and emetic episode duration. Immunohistochemistry demonstrated that motion alone did not induce c-fos expression in the brain. Paradoxically, mepyramine increased c-fos in brain areas regulating emesis control, and caused hypothermia; it also appeared to cause sedation and reduced the dominant frequency of slow waves. In conclusion, motion-induced emesis was associated with a disruption of GMA, respiration, and hypothermia. Mepyramine was a more efficacious anti-emetic than cetirizine, suggesting an important role of centrally-located H1 receptors. The ability of mepyramine to elevate c-fos provides a new perspective on how H1 receptors are involved in mechanisms of emesis control.
Keywords: Suncus murinus; gastric myoelectric activity; histamine H1 receptors; hypothermia; motion sickness; muscarinic receptors; respiration pattern.
Figures


Similar articles
-
The brain-penetrating, orally bioavailable, ghrelin receptor agonist HM01 ameliorates motion-induced emesis in Suncus murinus (house musk shrew).Br J Pharmacol. 2020 Apr;177(7):1635-1650. doi: 10.1111/bph.14924. Epub 2020 Feb 3. Br J Pharmacol. 2020. PMID: 31722444 Free PMC article.
-
Insights Into Acute and Delayed Cisplatin-Induced Emesis From a Microelectrode Array, Radiotelemetry and Whole-Body Plethysmography Study of Suncus murinus (House Musk Shrew).Front Pharmacol. 2021 Dec 3;12:746053. doi: 10.3389/fphar.2021.746053. eCollection 2021. Front Pharmacol. 2021. PMID: 34925008 Free PMC article.
-
Action of anti-tussive drugs on the emetic reflex of Suncus murinus (house musk shrew).Eur J Pharmacol. 2007 Mar 22;559(2-3):196-201. doi: 10.1016/j.ejphar.2006.12.008. Epub 2006 Dec 29. Eur J Pharmacol. 2007. PMID: 17254564
-
Neuropharmacology of motion sickness and emesis. A review.Acta Otolaryngol Suppl. 1993;501:10-5. doi: 10.3109/00016489309126205. Acta Otolaryngol Suppl. 1993. PMID: 8447218 Review.
-
The involvement of TRPV1 in emesis and anti-emesis.Temperature (Austin). 2015 May 21;2(2):258-76. doi: 10.1080/23328940.2015.1043042. eCollection 2015 Apr-Jun. Temperature (Austin). 2015. PMID: 27227028 Free PMC article. Review.
Cited by
-
Management of peripheral vertigo with antihistamines: New options on the horizon.Br J Clin Pharmacol. 2019 Oct;85(10):2255-2263. doi: 10.1111/bcp.14046. Epub 2019 Jul 22. Br J Clin Pharmacol. 2019. PMID: 31269270 Free PMC article. Review.
-
The brain-penetrating, orally bioavailable, ghrelin receptor agonist HM01 ameliorates motion-induced emesis in Suncus murinus (house musk shrew).Br J Pharmacol. 2020 Apr;177(7):1635-1650. doi: 10.1111/bph.14924. Epub 2020 Feb 3. Br J Pharmacol. 2020. PMID: 31722444 Free PMC article.
-
A History of Drug Discovery for Treatment of Nausea and Vomiting and the Implications for Future Research.Front Pharmacol. 2018 Sep 4;9:913. doi: 10.3389/fphar.2018.00913. eCollection 2018. Front Pharmacol. 2018. PMID: 30233361 Free PMC article. Review.
-
Insights Into Acute and Delayed Cisplatin-Induced Emesis From a Microelectrode Array, Radiotelemetry and Whole-Body Plethysmography Study of Suncus murinus (House Musk Shrew).Front Pharmacol. 2021 Dec 3;12:746053. doi: 10.3389/fphar.2021.746053. eCollection 2021. Front Pharmacol. 2021. PMID: 34925008 Free PMC article.
-
The effects of meclizine on motion sickness revisited.Br J Clin Pharmacol. 2020 Aug;86(8):1510-1518. doi: 10.1111/bcp.14257. Epub 2020 Mar 3. Br J Clin Pharmacol. 2020. PMID: 32077140 Free PMC article. Clinical Trial.
References
-
- Benavides J., Schoemaker H., Dana C., Claustre Y., Delahaye M., Prouteau M., et al. . (1995). In vivo and in vitro interaction of the novel selective histamine H1 receptor antagonist mizolastine with H1 receptors in the rodent. Arzneimittelforschung 45, 551–558. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

