Dynamic Acquisition and Loss of Dual-Obligate Symbionts in the Plant-Sap-Feeding Adelgidae (Hemiptera: Sternorrhyncha: Aphidoidea)
- PMID: 28659877
- PMCID: PMC5468457
- DOI: 10.3389/fmicb.2017.01037
Dynamic Acquisition and Loss of Dual-Obligate Symbionts in the Plant-Sap-Feeding Adelgidae (Hemiptera: Sternorrhyncha: Aphidoidea)
Abstract
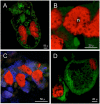
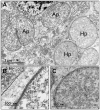
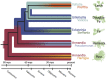
Sap-sucking insects typically engage in obligate relationships with symbiotic bacteria that play nutritional roles in synthesizing nutrients unavailable or in scarce supply from the plant-sap diets of their hosts. Adelgids are sap-sucking insects with complex life cycles that involve alternation between conifer tree species. While all adelgid species feed on spruce during the sexual phase of their life cycle, each adelgid species belongs to a major lineage that feeds on a distinct genus of conifers as their alternate host. Previous work on adelgid symbionts had discovered pairs of symbionts within each host species, and unusual diversity across the insect family, but left several open questions regarding the status of bacterial associates. Here, we explored the consistency of symbionts within and across adelgid lineages, and sought evidence for facultative vs. obligate symbiont status. Representative species were surveyed for symbionts using 16S ribosomal DNA gene sequencing, confirming that different symbiont pairs were consistently present within each major adelgid lineage. Several approaches were used to establish whether symbionts exhibited characteristics of long-term, obligate mutualists. Patterns of symbiont presence across adelgid species and diversification with host insects suggested obligate relationships. Fluorescent in situ hybridization and electron microscopy localized symbionts to bacteriocyte cells within the bacteriome of each species (with one previously known exception), and detection of symbionts in eggs indicated their vertical transmission. Common characteristics of long-term obligate symbionts, such as nucleotide compositional bias and pleomorphic symbiont cell shape were also observed. Superimposing microbial symbionts on the adelgid phylogeny revealed a dynamic pattern of symbiont gains and losses over a relatively short period of time compared to other symbionts associated with sap-sucking insects, with each adelgid species possessing an older, "senior" symbiont and a younger "junior" symbiont. A hypothesis relating adelgid life cycles to relaxed constraints on symbionts is proposed, with the degradation of senior symbionts and repeated acquisition of more junior symbionts creating opportunities for repeated colonization of new alternate-conifer hosts by adelgids.
Keywords: bacterial symbionts; complex life cycles; dual symbionts; host alternation; insects; symbiont replacements.
Figures



Similar articles
-
Transitional genomes and nutritional role reversals identified for dual symbionts of adelgids (Aphidoidea: Adelgidae).ISME J. 2022 Mar;16(3):642-654. doi: 10.1038/s41396-021-01102-w. Epub 2021 Sep 10. ISME J. 2022. PMID: 34508228 Free PMC article.
-
Co-evolution and symbiont replacement shaped the symbiosis between adelgids (Hemiptera: Adelgidae) and their bacterial symbionts.Environ Microbiol. 2012 May;14(5):1284-95. doi: 10.1111/j.1462-2920.2012.02712.x. Epub 2012 Feb 24. Environ Microbiol. 2012. PMID: 22364314
-
Partnering With a Pest: Genomes of Hemlock Woolly Adelgid Symbionts Reveal Atypical Nutritional Provisioning Patterns in Dual-Obligate Bacteria.Genome Biol Evol. 2018 Jun 1;10(6):1607-1621. doi: 10.1093/gbe/evy114. Genome Biol Evol. 2018. PMID: 29860412 Free PMC article.
-
Biology and evolution of adelgidae.Annu Rev Entomol. 2007;52:325-49. doi: 10.1146/annurev.ento.52.110405.091303. Annu Rev Entomol. 2007. PMID: 17163799 Review.
-
The nutritional dimension of facultative bacterial symbiosis in aphids: Current status and methodological considerations for future research.Curr Res Insect Sci. 2023 Dec 20;5:100070. doi: 10.1016/j.cris.2023.100070. eCollection 2024. Curr Res Insect Sci. 2023. PMID: 38222793 Free PMC article. Review.
Cited by
-
Microbiome analyses of 12 psyllid species of the family Psyllidae identified various bacteria including Fukatsuia and Serratia symbiotica, known as secondary symbionts of aphids.BMC Microbiol. 2022 Jan 7;22(1):15. doi: 10.1186/s12866-021-02429-2. BMC Microbiol. 2022. PMID: 34996376 Free PMC article.
-
Whole-genome sequence of the Cooley spruce gall adelgid, Adelges cooleyi (Hemiptera: Sternorrhyncha: Adelgidae).G3 (Bethesda). 2023 Dec 29;14(1):jkad224. doi: 10.1093/g3journal/jkad224. G3 (Bethesda). 2023. PMID: 37766465 Free PMC article.
-
PacBio Hi-Fi genome assembly of Sipha maydis, a model for the study of multipartite mutualism in insects.Sci Data. 2024 May 4;11(1):450. doi: 10.1038/s41597-024-03297-x. Sci Data. 2024. PMID: 38704391 Free PMC article.
-
Phylogenomics Identifies an Ancestral Burst of Gene Duplications Predating the Diversification of Aphidomorpha.Mol Biol Evol. 2020 Mar 1;37(3):730-756. doi: 10.1093/molbev/msz261. Mol Biol Evol. 2020. PMID: 31702774 Free PMC article.
-
Host relatedness influences the composition of aphid microbiomes.Environ Microbiol Rep. 2019 Dec;11(6):808-816. doi: 10.1111/1758-2229.12795. Epub 2019 Oct 20. Environ Microbiol Rep. 2019. PMID: 31573138 Free PMC article.
References
-
- Annand P. N. (1928). A Contribution toward a Monograph of the Adelginae (Phylloxeridae) of North America. Palo Alto CA: Stanford University Press.
-
- Auclair J. L. (1963). Aphid feeding and nutrition. Annu. Rev. Entomol. 8 439–490. 10.1146/annurev.en.08.010163.002255 - DOI
-
- Balch R. E., Underwood G. R. (1950). The life-history of Pineus pinifoliae (Fitch) (Homoptera: Phylloxeridae) and its effect on white pine. Can. Entomol. 82 117–123. 10.4039/Ent82117-6 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

