A Novel Method to Titrate Herpes Simplex Virus-1 (HSV-1) Using Laser-Based Scanning of Near-Infrared Fluorophores Conjugated Antibodies
- PMID: 28659899
- PMCID: PMC5469900
- DOI: 10.3389/fmicb.2017.01085
A Novel Method to Titrate Herpes Simplex Virus-1 (HSV-1) Using Laser-Based Scanning of Near-Infrared Fluorophores Conjugated Antibodies
Abstract
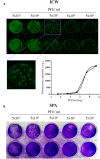
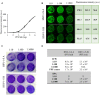
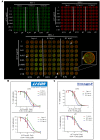
Among several strategies used for Herpes simplex virus (HSV) detection in biological specimens, standard plaque assay (SPA) remains the most reliable method to evaluate virus infectivity and quantify viral replication. However, it is a manual procedure, thereby affected by operator subjectivity, and it may be particularly laborious for multiple sample analysis. Here we describe an innovative method to perform the titration of HSV type 1 (HSV-1) in different samples, using the "In-Cell WesternTM" Assay (ICW) from LI-COR, a quantitative immunofluorescence assay that exploits laser-based scanning of near infrared (NIR). In particular, we employed NIR-immunodetection of viral proteins to monitor foci of HSV-1 infection in cell monolayers, and exploited an automated detection of their fluorescence intensity to evaluate virus titre. This innovative method produced similar and superimposable values compared to SPA, but it is faster and can be performed in 96 well plate, thus allowing to easily and quickly analyze and quantify many samples in parallel. These features make our method particularly suitable for the screening and characterization of antiviral compounds, as we demonstrated by testing acyclovir (ACV), the main anti-HSV-1 drug. Moreover, we developed a new data analysis system that allowed to overcome potential bias due to unspecific florescence signals, thus improving data reproducibility. Overall, our method may represents a useful tool for both clinical and research purposes.
Keywords: HSV-1; antivirals; herpes simplex virus; immunostaining; near-infrared fluorescence; plaque assay; virus titration.
Figures
References
-
- Civitelli L., Panella S., Marcocci M. E., De Petris A., Garzoli S., Pepi F., et al. (2014). In vitro inhibition of herpes simplex virus type 1 replication by Mentha suaveolens essential oil and its main component piperitenone oxide. Phytomedicine 21 857–865. 10.1016/j.phymed.2014.01.013 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

