Paradoxical Effect of Nonalcoholic Red Wine Polyphenol Extract, Provinols™, in the Regulation of Cyclooxygenases in Vessels from Zucker Fatty Rats (fa/ fa)
- PMID: 28660008
- PMCID: PMC5474272
- DOI: 10.1155/2017/8536910
Paradoxical Effect of Nonalcoholic Red Wine Polyphenol Extract, Provinols™, in the Regulation of Cyclooxygenases in Vessels from Zucker Fatty Rats (fa/ fa)
Abstract
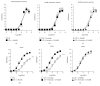
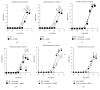
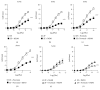
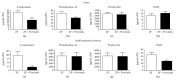
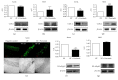
The aim of this work was to study the vascular effects of dietary supplementation of a nonalcoholic red wine polyphenol extract, Provinols, in Zucker fatty (ZF) obese rats. ZF or lean rats received diet supplemented or not with Provinols for 8 weeks. Vasoconstriction in response to phenylephrine (Phe) was then assessed in small mesenteric arteries (SMA) and the aorta with emphasis on the contribution of cyclooxygenases (COX). Although no difference in vasoconstriction was observed between ZF and lean rats both in SMA and the aorta, Provinols affected the contribution of COX-derived vasoconstrictor agents. The nonselective COX inhibitor, indomethacin, reduced vasoconstriction in vessels from both groups; however, lower efficacy was observed in Provinols-treated rats. This was associated with a reduction in thromboxane-A2 and 8-isoprostane release. The selective COX-2 inhibitor, NS398, reduced to the same extent vasoconstriction in aortas from ZF and Provinols-treated ZF rats. However, NS398 reduced response to Phe only in SMA from ZF rats. This was associated with a reduction in 8-isoprostane and prostaglandin-E release. Paradoxically, Provinols decreased COX-2 expression in the aorta, while it increased its expression in SMA. We provide here evidence of a subtle and paradoxical regulation of COX pathway by Provinols vessels from obese rats to maintain vascular tone within a physiological range.
Figures
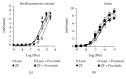
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

