The rise of 3-d single-ion magnets in molecular magnetism: towards materials from molecules?
- PMID: 28660017
- PMCID: PMC5477015
- DOI: 10.1039/c5sc03224e
The rise of 3-d single-ion magnets in molecular magnetism: towards materials from molecules?
Abstract
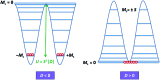
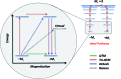
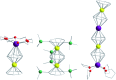
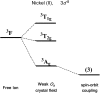
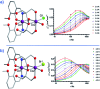
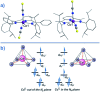
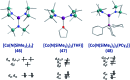
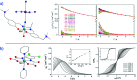
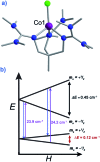
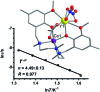
Single-molecule magnets (SMMs) that contain one spin centre (so-called single-ion magnets) theoretically represent the smallest possible unit for spin-based electronic devices. The realisation of this and related technologies, depends on first being able to design systems with sufficiently large energy barriers to magnetisation reversal, Ueff, and secondly, on being able to organise these molecules into addressable arrays. In recent years, significant progress has been made towards the former goal - principally as a result of efforts which have been directed towards studying complexes based on highly anisotropic lanthanide ions, such as Tb(iii) and Dy(iii). Since 2013 however, and the remarkable report by Long and co-workers of a linear Fe(i) system exhibiting Ueff = 325 K, single-ion systems of transition metals have undergone something of a renaissance in the literature. Not only do they have important lessons to teach us about anisotropy and relaxation dynamics in the quest to enhance Ueff, the ability to create strongly coupled spin systems potentially offers access to a whole of host of 1, 2 and 3-dimensional materials with interesting structural and physical properties. This perspective summarises recent progress in this rapidly expanding sub-genre of molecular magnetism from the viewpoint of the synthetic chemist, with a particular focus on the lessons that have so far been learned from single-ion magnets of the d-block, and, the future research directions which we feel are likely to emerge in the coming years.
Figures



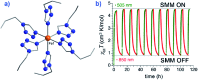


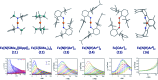
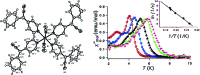
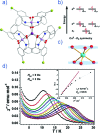







References
-
- Buschowand K. H. J. and de Boer F. R., Physics of Magnetic Materials, World Scientific, 1985.
-
- Craig G. A., Murrie M. Chem. Soc. Rev. 2015;44:2135. - PubMed
- Gómez-Coca S., Aravena D., Morales R., Ruiz E. Coord. Chem. Rev. 2015;290:379–392.
-
- Bogani L., Wernsdorfer W. Nat. Mater. 2008;7:179. - PubMed
-
- Sessoli R., Gatteschi D., Caneschi A., Novak M. A. Nature. 1993;365:141.
-
- Furrer A., Waldmann O. Rev. Mod. Phys. 2013;85:367.
- Timco G. A., McInnes E. J. L., Winpenny R. E. P. Chem. Soc. Rev. 2013;42:1796. - PubMed
- Timco G. A., Faust T. B., Tuna F., Winpenny R. E. P. Chem. Soc. Rev. 2011;40:3067. - PubMed
- Santini P., Carretta S., Troiani F., Amoretti G. Phys. Rev. Lett. 2011;107:230502. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

