Dedifferentiated Chondrocytes in Composite Microfibers As Tool for Cartilage Repair
- PMID: 28660185
- PMCID: PMC5468460
- DOI: 10.3389/fbioe.2017.00035
Dedifferentiated Chondrocytes in Composite Microfibers As Tool for Cartilage Repair
Abstract
Tissue engineering (TE) approaches using biomaterials have gain important roles in the regeneration of cartilage. This paper describes the production by microfluidics of alginate-based microfibers containing both extracellular matrix (ECM)-derived biomaterials and chondrocytes. As ECM components gelatin or decellularized urinary bladder matrix (UBM) were investigated. The effectiveness of the composite microfibers has been tested to modulate the behavior and redifferentiation of dedifferentiated chondrocytes. The complete redifferentiation, at the single-cell level, of the chondrocytes, without cell aggregate formation, was observed after 14 days of cell culture. Specific chondrogenic markers and high cellular secretory activity was observed in embedded cells. Notably, no sign of collagen type 10 deposition was determined. The obtained data suggest that dedifferentiated chondrocytes regain a functional chondrocyte phenotype when embedded in appropriate 3D scaffold based on alginate plus gelatin or UBM. The proposed scaffolds are indeed valuable to form a cellular microenvironment mimicking the in vivo ECM, opening the way to their use in cartilage TE.
Keywords: cartilage tissue engineering; chondrocytes; extracellular matrix-derived biomaterials; gene expression; microfibers.
Figures
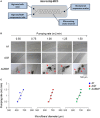




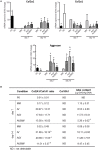

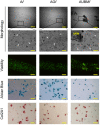
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

