Transforming Growth Factor-β2 Downregulates Major Histocompatibility Complex (MHC) I and MHC II Surface Expression on Equine Bone Marrow-Derived Mesenchymal Stem Cells Without Altering Other Phenotypic Cell Surface Markers
- PMID: 28660198
- PMCID: PMC5466990
- DOI: 10.3389/fvets.2017.00084
Transforming Growth Factor-β2 Downregulates Major Histocompatibility Complex (MHC) I and MHC II Surface Expression on Equine Bone Marrow-Derived Mesenchymal Stem Cells Without Altering Other Phenotypic Cell Surface Markers
Abstract
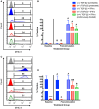
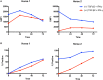
Allogeneic mesenchymal stem cells (MSCs) are a promising cell source for treating musculoskeletal injuries in horses. Effective and safe allogeneic therapy may be hindered, however, by recipient immune recognition and rejection of major histocompatibility complex (MHC)-mismatched MSCs. Development of strategies to prevent immune rejection of MHC-mismatched MSCs in vivo is necessary to enhance cell survival and potentially increase the efficacy and safety of allogeneic MSC therapy. The purposes of this study were to evaluate if transforming growth factor-β2 (TGF-β2) downregulated MHC expression on equine MSCs and to determine if TGF-β2 treatment altered the phenotype of MSCs. Equine bone marrow-derived MSCs from 12 horses were treated with 1, 5, or 10 ng/ml TGF-β2 from initial isolation until MHC expression analysis. TGF-β2-treated MSCs had reduced MHC I and MHC II surface expression compared to untreated controls. TGF-β2 treatment also partially blocked IFN-γ-induced upregulation of MHC I and MHC II. Constitutive and IFN-γ-induced MHC I and MHC II expression on equine MSCs was dynamic and highly variable, and the effect of TGF-β2 was significantly dependent on the donor animal and baseline MHC expression. TGF-β2 treatment did not appear to change morphology, surface marker expression, MSC viability, or secretion of TGF-β1, but did significantly increase the number of cells obtained from culture. These results indicate that TGF-β2 treatment has promise for regulating MHC expression on MSCs to facilitate allogeneic therapy, but further work is needed to maintain MHC stability when exposed to an inflammatory stimulus.
Keywords: IFN-γ; allogeneic; equine; major histocompatibility complex; mesenchymal stem cell; transforming growth factor-β2.
Figures







References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

