Expression, Purification and Crystallisation of the Adenosine A2A Receptor Bound to an Engineered Mini G Protein
- PMID: 28660236
- PMCID: PMC5484405
- DOI: 10.21769/BioProtoc.2234
Expression, Purification and Crystallisation of the Adenosine A2A Receptor Bound to an Engineered Mini G Protein
Abstract
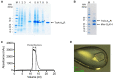
G protein-coupled receptors (GPCRs) promote cytoplasmic signalling by activating heterotrimeric G proteins in response to extracellular stimuli such as light, hormones and nucleosides. Structure determination of GPCR-G protein complexes is central to understanding the precise mechanism of signal transduction. However, these complexes are challenging targets for structural studies due to their conformationally dynamic and inherently transient nature. We recently developed an engineered G protein, mini-Gs, which addressed these problems and allowed the formation of a stable GPCR-G protein complex. Mini-Gs facilitated the structure determination of the human adenosine A2A receptor (A2AR) in its G protein-bound conformation at 3.4 Å resolution. Here, we describe a step by step protocol for the expression and purification of A2AR, and crystallisation of the A2AR-mini-Gs complex.
Keywords: A2AR; Active state; Adenosine A2A receptor; G protein complex; G protein-coupled receptor; GPCR; Mini G protein; Mini-Gs.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

