Systemic Nicotine Increases Gain and Narrows Receptive Fields in A1 via Integrated Cortical and Subcortical Actions
- PMID: 28660244
- PMCID: PMC5480142
- DOI: 10.1523/ENEURO.0192-17.2017
Systemic Nicotine Increases Gain and Narrows Receptive Fields in A1 via Integrated Cortical and Subcortical Actions
Abstract
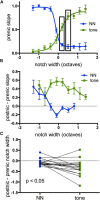
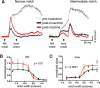
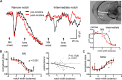
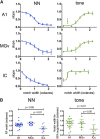
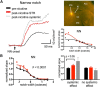
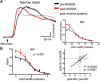
Nicotine enhances sensory and cognitive processing via actions at nicotinic acetylcholine receptors (nAChRs), yet the precise circuit- and systems-level mechanisms remain unclear. In sensory cortex, nicotinic modulation of receptive fields (RFs) provides a model to probe mechanisms by which nAChRs regulate cortical circuits. Here, we examine RF modulation in mouse primary auditory cortex (A1) using a novel electrophysiological approach: current-source density (CSD) analysis of responses to tone-in-notched-noise (TINN) acoustic stimuli. TINN stimuli consist of a tone at the characteristic frequency (CF) of the recording site embedded within a white noise stimulus filtered to create a spectral "notch" of variable width centered on CF. Systemic nicotine (2.1 mg/kg) enhanced responses to the CF tone and to narrow-notch stimuli, yet reduced the response to wider-notch stimuli, indicating increased response gain within a narrowed RF. Subsequent manipulations showed that modulation of cortical RFs by systemic nicotine reflected effects at several levels in the auditory pathway: nicotine suppressed responses in the auditory midbrain and thalamus, with suppression increasing with spectral distance from CF so that RFs became narrower, and facilitated responses in the thalamocortical pathway, while nicotinic actions within A1 further contributed to both suppression and facilitation. Thus, multiple effects of systemic nicotine integrate along the ascending auditory pathway. These actions at nAChRs in cortical and subcortical circuits, which mimic effects of auditory attention, likely contribute to nicotinic enhancement of sensory and cognitive processing.
Keywords: A1; acetylcholine; auditory cortex; mouse; nicotine; receptive field.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
