The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity
- PMID: 28660319
- PMCID: PMC5645435
- DOI: 10.1007/s00262-017-2034-7
The clinical efficacy of first-generation carcinoembryonic antigen (CEACAM5)-specific CAR T cells is limited by poor persistence and transient pre-conditioning-dependent respiratory toxicity
Abstract
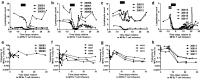
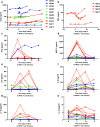
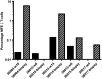
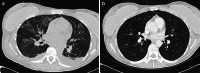
The primary aim of this clinical trial was to determine the feasibility of delivering first-generation CAR T cell therapy to patients with advanced, CEACAM5+ malignancy. Secondary aims were to assess clinical efficacy, immune effector function and optimal dose of CAR T cells. Three cohorts of patients received increasing doses of CEACAM5+-specific CAR T cells after fludarabine pre-conditioning plus systemic IL2 support post T cell infusion. Patients in cohort 4 received increased intensity pre-conditioning (cyclophosphamide and fludarabine), systemic IL2 support and CAR T cells. No objective clinical responses were observed. CAR T cell engraftment in patients within cohort 4 was significantly higher. However, engraftment was short-lived with a rapid decline of systemic CAR T cells within 14 days. Patients in cohort 4 had transient, acute respiratory toxicity which, in combination with lack of prolonged CAR T cell persistence, resulted in the premature closure of the trial. Elevated levels of systemic IFNγ and IL-6 implied that the CEACAM5-specific T cells had undergone immune activation in vivo but only in patients receiving high-intensity pre-conditioning. Expression of CEACAM5 on lung epithelium may have resulted in this transient toxicity. Raised levels of serum cytokines including IL-6 in these patients implicate cytokine release as one of several potential factors exacerbating the observed respiratory toxicity. Whilst improved CAR designs and T cell production methods could improve the systemic persistence and activity, methods to control CAR T 'on-target, off-tissue' toxicity are required to enable a clinical impact of this approach in solid malignancies.
Keywords: CEA; Chimeric antigen receptor; Persistence; T cells; Toxicity.
Conflict of interest statement
David Gilham, Ryan Guest and Robert Hawkins are all co-founders and shareholders of Cellular Therapeutics Ltd. Robert Hawkins also declares research funding: Pfizer, Bristol-Myers Squibb (BMS), Novartis, Glaxosmithkline (GSK); ad hoc advisory roles: Pfizer, BMS, Ipsen, Celegene, AstraZeneca (AZ), Novartis and GSK. Fiona Thistlethwaite declares ad hoc advisory roles: BMS; speaker honoraria BMS; Project support: Pfizer, Novartis; travel support: Ipsen, BMS. All other authors declare that they have no conflict of interest.
Figures
References
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33:540–549. doi: 10.1200/JCO.2014.56.2025. - DOI - PMC - PubMed
-
- Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

