Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data
- PMID: 28661008
- PMCID: PMC6481892
- DOI: 10.1002/14651858.CD011412.pub2
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data
Update in
-
Antiepileptic drug monotherapy for epilepsy: a network meta-analysis of individual participant data.Cochrane Database Syst Rev. 2017 Dec 15;12(12):CD011412. doi: 10.1002/14651858.CD011412.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2022 Apr 1;4:CD011412. doi: 10.1002/14651858.CD011412.pub4. PMID: 29243813 Free PMC article. Updated.
Abstract
Background: Epilepsy is a common neurological condition with a worldwide prevalence of around 1%. Approximately 60% to 70% of people with epilepsy will achieve a longer-term remission from seizures, and most achieve that remission shortly after starting antiepileptic drug treatment. Most people with epilepsy are treated with a single antiepileptic drug (monotherapy) and current guidelines from the National Institute for Health and Care Excellence (NICE) in the United Kingdom for adults and children recommend carbamazepine or lamotrigine as first-line treatment for partial onset seizures and sodium valproate for generalised onset seizures; however a range of other antiepileptic drug (AED) treatments are available, and evidence is needed regarding their comparative effectiveness in order to inform treatment choices.
Objectives: To compare the time to withdrawal of allocated treatment, remission and first seizure of 10 AEDs (carbamazepine, phenytoin, sodium valproate, phenobarbitone, oxcarbazepine, lamotrigine, gabapentin, topiramate, levetiracetam, zonisamide) currently used as monotherapy in children and adults with partial onset seizures (simple partial, complex partial or secondary generalised) or generalised tonic-clonic seizures with or without other generalised seizure types (absence, myoclonus).
Search methods: We searched the following databases: Cochrane Epilepsy's Specialised Register, CENTRAL, MEDLINE and SCOPUS, and two clinical trials registers. We handsearched relevant journals and contacted pharmaceutical companies, original trial investigators, and experts in the field. The date of the most recent search was 27 July 2016.
Selection criteria: We included randomised controlled trials of a monotherapy design in adults or children with partial onset seizures or generalised onset tonic-clonic seizures (with or without other generalised seizure types).
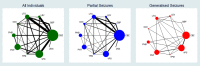
Data collection and analysis: This was an individual participant data (IPD) review and network meta-analysis. Our primary outcome was 'time to withdrawal of allocated treatment', and our secondary outcomes were 'time to achieve 12-month remission', 'time to achieve six-month remission', 'time to first seizure post-randomisation', and 'occurrence of adverse events'. We presented all time-to-event outcomes as Cox proportional hazard ratios (HRs) with 95% confidence intervals (CIs). We performed pairwise meta-analysis of head-to-head comparisons between drugs within trials to obtain 'direct' treatment effect estimates and we performed frequentist network meta-analysis to combine direct evidence with indirect evidence across the treatment network of 10 drugs. We investigated inconsistency between direct estimates and network meta-analysis via node splitting. Due to variability in methods and detail of reporting adverse events, we have not performed an analysis. We have provided a narrative summary of the most commonly reported adverse events.
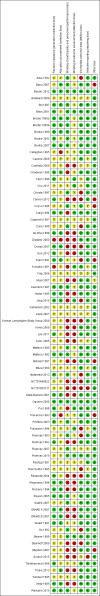
Main results: IPD was provided for at least one outcome of this review for 12,391 out of a total of 17,961 eligible participants (69% of total data) from 36 out of the 77 eligible trials (47% of total trials). We could not include IPD from the remaining 41 trials in analysis for a variety of reasons, such as being unable to contact an author or sponsor to request data, data being lost or no longer available, cost and resources required to prepare data being prohibitive, or local authority or country-specific restrictions.We were able to calculate direct treatment effect estimates for between half and two thirds of comparisons across the outcomes of the review, however for many of the comparisons, data were contributed by only a single trial or by a small number of participants, so confidence intervals of estimates were wide.Network meta-analysis showed that for the primary outcome 'Time to withdrawal of allocated treatment,' for individuals with partial seizures; levetiracetam performed (statistically) significantly better than both current first-line treatments carbamazepine and lamotrigine; lamotrigine performed better than all other treatments (aside from levetiracetam), and carbamazepine performed significantly better than gabapentin and phenobarbitone (high-quality evidence). For individuals with generalised onset seizures, first-line treatment sodium valproate performed significantly better than carbamazepine, topiramate and phenobarbitone (moderate- to high-quality evidence). Furthermore, for both partial and generalised onset seizures, the earliest licenced treatment, phenobarbitone seems to perform worse than all other treatments (moderate- to high-quality evidence).Network meta-analysis also showed that for secondary outcomes 'Time to 12-month remission of seizures' and 'Time to six-month remission of seizures,' few notable differences were shown for either partial or generalised seizure types (moderate- to high-quality evidence). For secondary outcome 'Time to first seizure,' for individuals with partial seizures; phenobarbitone performed significantly better than both current first-line treatments carbamazepine and lamotrigine; carbamazepine performed significantly better than sodium valproate, gabapentin and lamotrigine. Phenytoin also performed significantly better than lamotrigine (high-quality evidence). In general, the earliest licenced treatments (phenytoin and phenobarbitone) performed better than the other treatments for both seizure types (moderate- to high-quality evidence).Generally, direct evidence and network meta-analysis estimates (direct plus indirect evidence) were numerically similar and consistent with confidence intervals of effect sizes overlapping.The most commonly reported adverse events across all drugs were drowsiness/fatigue, headache or migraine, gastrointestinal disturbances, dizziness/faintness and rash or skin disorders.
Authors' conclusions: Overall, the high-quality evidence provided by this review supports current guidance (e.g. NICE) that carbamazepine and lamotrigine are suitable first-line treatments for individuals with partial onset seizures and also demonstrates that levetiracetam may be a suitable alternative. High-quality evidence from this review also supports the use of sodium valproate as the first-line treatment for individuals with generalised tonic-clonic seizures (with or without other generalised seizure types) and also demonstrates that lamotrigine and levetiracetam would be suitable alternatives to either of these first-line treatments, particularly for those of childbearing potential, for whom sodium valproate may not be an appropriate treatment option due to teratogenicity.
Conflict of interest statement
SJN was funded between 2011 and 2014 as part of a three‐year research programme, ‘Clinical and cost effectiveness of interventions for epilepsy in the National Health Service (NHS)’, which receives financial support from the National Institute of Health Research (NIHR).
JW was funded between 2011 and 2014 as part of a three‐year research programme, ‘Clinical and cost effectiveness of interventions for epilepsy in the National Health Service (NHS)’, which receives financial support from the National Institute of Health Research (NIHR).
AGM: a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is Theme Leader for Managing Complex Needs at NIHR CLAHRC NWC.
Figures














References
References to studies included in this review
-
- Aikia M, Kalviainen R, Sivenius J, Halonen T, Riekkinen PJ. Cognitive effects of oxcarbazepine and phenytoin monotherapy in newly diagnosed epilepsy: one year follow‐up. Epilepsy Research 1992;11(3):199‐203. - PubMed
-
- Baulac M, Brodie MJ, Patten A, Segieth J, Giorgi L. Efficacy and tolerability of zonisamide versus controlled‐release carbamazepine for newly diagnosed partial epilepsy: a phase 3, randomised, double‐blind, non‐inferiority trial. Lancet Neurology 2012;11(7):579‐88. - PubMed
-
- Bidabadi E. Comparison of the effects of phenobarbital versus carbamazepine as single drug therapy in partial seizure with secondary generalization in children. Epilepsia 2009;50(Suppl 10):167. Abstract no: p772 28th International Epilepsy Congress; Budapest 2009.
-
- Bill PA, Vigonius U, Pohlmann H, Guerreiro CA, Kochen S, Saffer D, et al. A double‐blind controlled clinical trial of oxcarbazepine versus phenytoin in adults with previously untreated epilepsy. Epilepsy Research 1997;27(3):195‐204. - PubMed
References to studies excluded from this review
-
- Albani F, Baruzzi A, Primo Study Group. Oxcarbazepine long‐term treatment retention in patients switched over from carbamazepine. Neurological Sciences 2006;27(3):173‐5. - PubMed
-
- Alsaadi TM, Thieman C, Zusman EE. Levetiracetam monotherapy for adults with localization‐related epilepsy. Epilepsy and Behavior 2002;3(5):471‐4. - PubMed
-
- Alsaadi TM, Shatzel A, Marquez AV, Jorgensen J, Farias S. Clinical experience of levetiracetam monotherapy for adults with epilepsy: 1‐year follow‐up study. Seizure 2005;14(2):139‐42. - PubMed
-
- Baxter L, Cheesbrough A. An open randomised comparison of Lamictal (lamotrigine) with physicians preferred choice of either valproate or carbamazepine as monotherapy in patients over 12 years of age with newly diagnosed epilepsy. Clinical summary report1998.
-
- Ben‐Menachem E. Preliminary efficacy of levetiracetam in monotherapy. Epileptic Disorders 2003;5(Suppl 1):S51‐5. - PubMed
References to studies awaiting assessment
-
- Chen YB, Hao YP, Hao XS, Liang D. Clinical efficacy of oxcarbazepine suspension in children with focal epilepsy. Zhongguo Dang Dai Er Ke Za Zhi 2013;15(5):340‐2. - PubMed
- Chen YB, Wang JT, Wang LJ, Liang D. Clinical observation of oxcarbazepine suspension monotherapy for 2 to 4‐year‐old patients newly diagnosed as partial epilepsy. Chinese Journal of Neurology 2012;45(10):730‐3.
-
- IRCT201202068943N1. Efficacy of oxcarbazepine and phenytoin in control of epilepsy in the elderly. www.irct.ir/searchresult.php?id=8943&number=1 (accessed 23 August 2016).
-
- Korean Zonisamide Study Group. Double‐blind, randomized, comparative clinical trial of zonisamide and carbamazepine as initial monotherapy in newly diagnosed epilepsy. Journal of Korean Epilepsy Society 1999;3(1):50‐7.
-
- NCT00154076. A multicenter comparative trial of zonisamide and topiramate as initial monotherapy in untreated epilepsies. ClinicalTrials.gov (accessed 23 August 2016).
-
- Park WS, Kim CW, Park SP, Kwon SH. Safety and efficacy of topiramate monotherapy in children with recent‐onset seizures. Journal of Korean Epilepsy Society 2001;5(1):65‐9.
References to ongoing studies
-
- ACTRN12615000556549. EpiNet‐First Trial 2: comparison of efficacy of levetiracetam and sodium valproate in people with previously untreated epilepsy who have generalised seizures. In: Auckland District Health B, editor. 2015 (accessed 23 August 2016).
-
- ACTRN12615000639527. EpiNet‐First Trial 3: comparison of efficacy of levetiracetam and lamotrigine in people with previously untreated epilepsy who have generalised seizures, and for whom sodium valproate is not deemed an acceptable anti‐epileptic drug. In: Auckland District Health B, editor. 2015 (accessed 23 August 2016).
-
- ACTRN12615000640505. EpiNet‐First Trial 4: comparison of efficacy of levetiracetam, lamotrigine and sodium valproate in people with previously untreated epilepsy who have unclassified seizures. In: Auckland District Health B, editor. 2015 Vol. (accessed 23 August 2016).
-
- ACTRN12615000641594. EpiNet‐First Trial 5: comparison of efficacy of levetiracetam and lamotrigine in people with previously untreated epilepsy who have unclassified seizures, and for whom sodium valproate is not deemed an acceptable anti‐epileptic drug. In: Auckland District Health B, editor. 2015 (accessed 23 Agust 2016).
-
- ACTRN12615000643572. EpiNet‐First Trial 1: comparison of efficacy of levetiracetam, lamotrigine and carbamazepine in people with previously untreated epilepsy who have focal seizures. In: Auckland District Health B, editor. 2015 (accessed 23 August 2016).
Additional references
-
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999;40(4):502‐6. - PubMed
-
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, Emde Boas W, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005‐2009. Epilepsia 2010;51:676‐85. - PubMed
-
- Bourgeois B, Beaumanoir A, Blajev B, Cruz N, Despland PA, Egli M, et al. Monotherapy with valproate in primary generalized epilepsies. Epilepsia 1987;28(Suppl 2):S8‐11. - PubMed
-
- Brodie MJ, Dichter MA. Antiepileptic drugs. New England Journal of Medicine 1996;334(3):168‐75. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

