Osteoblastic heparan sulfate glycosaminoglycans control bone remodeling by regulating Wnt signaling and the crosstalk between bone surface and marrow cells
- PMID: 28661485
- PMCID: PMC5520938
- DOI: 10.1038/cddis.2017.287
Osteoblastic heparan sulfate glycosaminoglycans control bone remodeling by regulating Wnt signaling and the crosstalk between bone surface and marrow cells
Erratum in
-
Correction: Osteoblastic heparan sulfate glycosaminoglycans control bone remodeling by regulating Wnt signaling and the crosstalk between bone surface and marrow cells.Cell Death Dis. 2018 Jul 16;9(8):788. doi: 10.1038/s41419-018-0830-4. Cell Death Dis. 2018. PMID: 30013102 Free PMC article.
Abstract
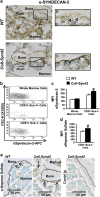
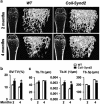
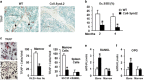
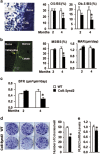
Stimulating bone formation is an important challenge for bone anabolism in osteoporotic patients or to repair bone defects. The osteogenic properties of matrix glycosaminoglycans (GAGs) have been explored; however, the functions of GAGs at the surface of bone-forming cells are less documented. Syndecan-2 is a membrane heparan sulfate proteoglycan that is associated with osteoblastic differentiation. We used a transgenic mouse model with high syndecan-2 expression in osteoblasts to enrich the bone surface with cellular GAGs. Bone mass was increased in these transgenic mice. Syndecan-2 overexpression reduced the expression of receptor activator of NF-kB ligand (RANKL) in bone marrow cells and strongly inhibited bone resorption. Osteoblast activity was not modified in the transgenic mice, but bone formation was decreased in 4-month-old transgenic mice because of reduced osteoblast number. Increased proteoglycan expression at the bone surface resulted in decreased osteoblastic and osteoclastic precursors in bone marrow. Indeed, syndecan-2 overexpression increased apoptosis of mesenchymal precursors within the bone marrow. However, syndecan-2 specifically promoted the vasculature characterized by high expression of CD31 and Endomucin in 6-week-old transgenic mice, but this was reduced in 12-week-old transgenic mice. Finally, syndecan-2 functions as an inhibitor of Wnt-β-catenin-T-cell factor signaling pathway, activating glycogen synthase kinase 3 and then decreasing the Wnt-dependent production of Wnt ligands and R-spondin. In conclusion, our results show that GAG supply may improve osteogenesis, but also interfere with the crosstalk between the bone surface and marrow cells, altering the supporting function of osteoblasts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Salbach-Hirsch J, Ziegler N, Thiele S, Moeller S, Schnabelrauch M, Hintze V et al. Sulfated glycosaminoglycans support osteoblast functions and concurrently suppress osteoclasts. J Cell Biochem 2014; 115: 1101–1111. - PubMed
-
- Salbach J, Rachner TD, Rauner M, Hempel U, Anderegg U, Franz S et al. Regenerative potential of glycosaminoglycans for skin and bone. J Mol Med 2012; 90: 625–635. - PubMed
-
- Banka S, Cain Sa, Carim S, Daly SB, Urquhart JE, Erdem G et al. Leri’s pleonosteosis, a congenital rheumatic disease, results from microduplication at 8q22.1 encompassing GDF6 and SDC2 and provides insight into systemic sclerosis pathogenesis. Ann Rheum Dis 2014; 74: 1249–1256. - PubMed
-
- Kingma SDK, Wagemans T, IJlst L, Bronckers ALJJ, van Kuppevelt TH, Everts V et al. Altered interaction and distribution of glycosaminoglycans and growth factors in mucopolysaccharidosis type I bone disease. Bone 2016; 88: 92–100. - PubMed
-
- Picke A-K, Salbach-Hirsch J, Hintze V, Rother S, Rauner M, Kascholke C et al. Sulfated hyaluronan improves bone regeneration of diabetic rats by binding sclerostin and enhancing osteoblast function. Biomaterials 2016; 96: 11–23. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

