The role of MAP2 kinases and p38 kinase in acute murine liver injury models
- PMID: 28661486
- PMCID: PMC5584575
- DOI: 10.1038/cddis.2017.295
The role of MAP2 kinases and p38 kinase in acute murine liver injury models
Abstract
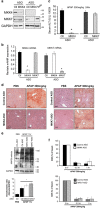
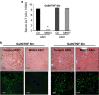
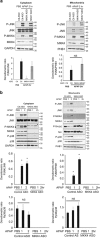
c-Jun N-terminal kinase (JNK) mediates hepatotoxicity through interaction of its phospho-activated form with a mitochondrial outer membrane protein, Sh3bp5 or Sab, leading to dephosphorylation of intermembrane Src and consequent impaired mitochondrial respiration and enhanced ROS release. ROS production from mitochondria activates MAP3 kinases, such as MLK3 and ASK1, which continue to activate a pathway to sustain JNK activation, and amplifies the toxic effect of acetaminophen (APAP) and TNF/galactosamine (TNF/GalN). Downstream of MAP3K, in various contexts MKK4 activates both JNK and p38 kinases and MKK7 activates only JNK. The relative role of MKK4 versus 7 in liver injury is largely unexplored, as is the potential role of p38 kinase, which might be a key mediator of toxicity in addition to JNK. Antisense oligonucleotides (ASO) to MKK4, MKK7 and p38 (versus scrambled control) were used for in vivo knockdown, and in some experiments PMH were used after in vivo knockdown. Mice were treated with APAP or TNF/GalN and injury assessed. MKK4 and MKK7 were expressed in liver and each was efficiently knocked down with two different ASOs. Massive liver injury and ALT elevation were abrogated by MKK4 but not MKK7 ASO pretreatment in both injury models. The protection was confirmed in PMH. Knockdown of MKK4 completely inhibited basal P-p38 in both cytoplasm and mitochondria. However, ALT levels and histologic injury in APAP-treated mice were not altered with p38 knockdown versus scrambled control. p38 knockdown significantly increased P-JNK levels in cytoplasm but not mitochondria after APAP treatment. In conclusion, MKK4 is the major MAP2K, which activates JNK in acute liver injury. p38, the other downstream target of MKK4, does not contribute to liver injury from APAP or TNF/galactosamine.
Conflict of interest statement
MA is an employee and shareholder of Ionis Pharmaceuticals. The remaining authors declare no conflict of interest.
Figures


Similar articles
-
Regulation of inflammatory arthritis by the upstream kinase mitogen activated protein kinase kinase 7 in the c-Jun N-terminal kinase pathway.Arthritis Res Ther. 2012 Feb 21;14(1):R38. doi: 10.1186/ar3750. Arthritis Res Ther. 2012. PMID: 22353730 Free PMC article.
-
Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation.Gastroenterology. 2008 Oct;135(4):1311-21. doi: 10.1053/j.gastro.2008.07.006. Epub 2008 Jul 9. Gastroenterology. 2008. PMID: 18700144
-
c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity.Gastroenterology. 2006 Jul;131(1):165-78. doi: 10.1053/j.gastro.2006.03.045. Gastroenterology. 2006. PMID: 16831600
-
Pathophysiological significance of c-jun N-terminal kinase in acetaminophen hepatotoxicity.Expert Opin Drug Metab Toxicol. 2015;11(11):1769-79. doi: 10.1517/17425255.2015.1071353. Epub 2015 Jul 20. Expert Opin Drug Metab Toxicol. 2015. PMID: 26190663 Free PMC article. Review.
-
Acetaminophen hepatotoxicity: A mitochondrial perspective.Adv Pharmacol. 2019;85:195-219. doi: 10.1016/bs.apha.2019.01.007. Epub 2019 Feb 21. Adv Pharmacol. 2019. PMID: 31307587 Free PMC article. Review.
Cited by
-
Thioredoxin Prevents Loss of UCP2 in Hyperoxia via MKK4-p38 MAPK-PGC1α Signaling and Limits Oxygen Toxicity.Am J Respir Cell Mol Biol. 2022 Mar;66(3):323-336. doi: 10.1165/rcmb.2021-0219OC. Am J Respir Cell Mol Biol. 2022. PMID: 34890296 Free PMC article.
-
New insights into the role and mechanism of c-Jun-N-terminal kinase signaling in the pathobiology of liver diseases.Hepatology. 2018 May;67(5):2013-2024. doi: 10.1002/hep.29689. Epub 2018 Apr 6. Hepatology. 2018. PMID: 29194686 Free PMC article. Review.
-
Nrf2 as a therapeutic target in acetaminophen hepatotoxicity: A case study with sulforaphane.J Biochem Mol Toxicol. 2023 Dec;37(12):e23505. doi: 10.1002/jbt.23505. Epub 2023 Aug 20. J Biochem Mol Toxicol. 2023. PMID: 37598316 Free PMC article.
-
Expression of mitochondrial membrane-linked SAB determines severity of sex-dependent acute liver injury.J Clin Invest. 2019 Dec 2;129(12):5278-5293. doi: 10.1172/JCI128289. J Clin Invest. 2019. PMID: 31487267 Free PMC article.
-
Mechanism and Therapeutic Targets of c-Jun-N-Terminal Kinases Activation in Nonalcoholic Fatty Liver Disease.Biomedicines. 2022 Aug 20;10(8):2035. doi: 10.3390/biomedicines10082035. Biomedicines. 2022. PMID: 36009582 Free PMC article. Review.
References
-
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A et al. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology 2008; 135: 1311–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

