Endochondral Ossification in Critical-Sized Bone Defects via Readily Implantable Scaffold-Free Stem Cell Constructs
- PMID: 28661587
- PMCID: PMC5689752
- DOI: 10.1002/sctm.16-0222
Endochondral Ossification in Critical-Sized Bone Defects via Readily Implantable Scaffold-Free Stem Cell Constructs
Abstract
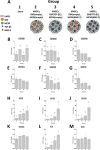
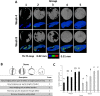
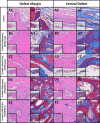
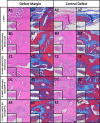
The growing socioeconomic burden of musculoskeletal injuries and limitations of current therapies have motivated tissue engineering approaches to generate functional tissues to aid in defect healing. A readily implantable scaffold-free system comprised of human bone marrow-derived mesenchymal stem cells embedded with bioactive microparticles capable of controlled delivery of transforming growth factor-beta 1 (TGF-β1) and bone morphogenetic protein-2 (BMP-2) was engineered to guide endochondral bone formation. The microparticles were formulated to release TGF-β1 early to induce cartilage formation and BMP-2 in a more sustained manner to promote remodeling into bone. Cell constructs containing microparticles, empty or loaded with one or both growth factors, were implanted into rat critical-sized calvarial defects. Micro-computed tomography and histological analyses after 4 weeks showed that microparticle-incorporated constructs with or without growth factor promoted greater bone formation compared to sham controls, with the greatest degree of healing with bony bridging resulting from constructs loaded with BMP-2 and TGF-β1. Importantly, bone volume fraction increased significantly from 4 to 8 weeks in defects treated with both growth factors. Immunohistochemistry revealed the presence of types I, II, and X collagen, suggesting defect healing via endochondral ossification in all experimental groups. The presence of vascularized red bone marrow provided strong evidence for the ability of these constructs to stimulate angiogenesis. This system has great translational potential as a readily implantable combination therapy that can initiate and accelerate endochondral ossification in vivo. Importantly, construct implantation does not require prior lengthy in vitro culture for chondrogenic cell priming with growth factors that is necessary for current scaffold-free combination therapies. Stem Cells Translational Medicine 2017;6:1644-1659.
Keywords: Adult stem cells; Bone tissue engineering; Growth factor delivery; Microparticles; Regenerative medicine; Self-assembled sheets.
© 2017 The Authors Stem Cells Translational Medicine published by Wiley Periodicals, Inc. on behalf of AlphaMed Press.
Figures


References
-
- Chen SS, Fitzgerald W, Zimmerberg J et al. Cell‐cell and cell‐extracellular matrix interactions regulate embryonic stem cell differentiation. Stem Cells 2007;25:553–561. - PubMed
-
- Levy‐Mishali M, Zoldan J, Levenberg S. Effect of scaffold stiffness on myoblast differentiation. Tissue Eng Part A 2009;15:935–944. - PubMed
-
- Reis RL, Cohn D. Polymer based systems on tissue engineering, replacement and regeneration. NATO Science Series, Series II: Mathematics, Physics and Chemistry, 1568‐2609 86. Dordrecht: Springer Netherlands: Imprint: Springer, 2002:1 online resource (440 pages).
-
- Langer RS, Lanza RP, Vacanti J. Principles of Tissue Engineering: Elsevier Inc., 2014: 1 online resource (xlviii, 1887 pages). https://www.elsevier.com/books/principles-of-tissue-engineering/lanza/97...

