Participant-Reported Symptoms and Their Effect on Long-Term Adherence in the International Breast Cancer Intervention Study I (IBIS I)
- PMID: 28661758
- PMCID: PMC5549455
- DOI: 10.1200/JCO.2016.71.7439
Participant-Reported Symptoms and Their Effect on Long-Term Adherence in the International Breast Cancer Intervention Study I (IBIS I)
Erratum in
-
Errata.J Clin Oncol. 2018 Jan 20;36(3):304. doi: 10.1200/JCO.2017.77.1956. J Clin Oncol. 2018. PMID: 29342375 Free PMC article. No abstract available.
Abstract
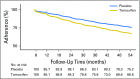
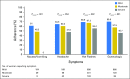
Purpose To assess the role of participant-reported symptoms on long-term adherence to preventive therapy in the United Kingdom sample of the International Breast Cancer Intervention Study (IBIS-I). IBIS-I was a randomized controlled trial that investigated the effectiveness of tamoxifen in reducing the risk of breast cancer among women at increased risk of the disease. Participants and Methods Women were randomly assigned to tamoxifen versus placebo (20 mg/day; n = 4,279). After 456 exclusions, 3,823 women were included in this analysis. Adherence (< 4.5 years or ≥ 4.5 years) was calculated using data from six monthly clinical visits. Analyses were adjusted for age, Tyrer-Cuzick risk, smoking, use of hormone replacement therapy, menopausal status, baseline menopausal symptoms, and treatment. Results Overall, 69.7% of women were adherent for at least 4.5 years (tamoxifen: 65.2% v placebo: 74.0%; P < .001). Differences in adherence between treatment arms were observed from 12 months onward (all P < .01) and were largest at 54 months. Dropout rates were highest in the first 12 to 18 months and decreased thereafter. Women reporting nausea/vomiting were less likely to be adherent in both the tamoxifen (odds ratio [OR], 0.57; 95% CI, 0.37 to 0.86; P = .007) and placebo (OR, 0.58; 95% CI, 0.37 to 0.93; P = .023) arms. Headaches were associated with adherence only in the placebo arm (OR, 0.62; 95% CI, 0.42 to 0.91; P = .016), whereas gynecologic symptoms were significant only in the tamoxifen arm (OR, 0.77; 95% CI, 0.62 to 0.97; P = .024). Effect sizes for each symptom on adherence were not significantly different between the treatment groups ( P > .05). In both treatment arms, we observed significant trends for lower adherence with increasing severity for all symptoms ( P < .01) except headaches ( P = .054). Conclusion In the IBIS-I trial, experiencing predefined symptoms in the first 6 months reduced long-term adherence. Effects were similar between treatment arms, suggesting that women were attributing age-related symptoms to preventive therapy. Interventions were required to support symptom management.
Figures


References
-
- Forouzanfar MH, Foreman KJ, Delossantos AM, et al. : Breast and cervical cancer in 187 countries between 1980 and 2010: A systematic analysis. Lancet 378:1461-1484, 2011 - PubMed
-
- Cancer Research UK: Breast cancer statistics. 2015. http://www.cancerresearchuk.org/health-professional/cancer-statistics/st....
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. : Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359-E386, 2015 - PubMed
-
- DeSantis C, Ma J, Bryan L, et al. : Breast cancer statistics, 2013. CA Cancer J Clin 64:52-62, 2014 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

