CaMKII Activation Promotes Cardiac Electrical Remodeling and Increases the Susceptibility to Arrhythmia Induction in High-fat Diet-Fed Mice With Hyperlipidemia Conditions
- PMID: 28662005
- PMCID: PMC5642343
- DOI: 10.1097/FJC.0000000000000512
CaMKII Activation Promotes Cardiac Electrical Remodeling and Increases the Susceptibility to Arrhythmia Induction in High-fat Diet-Fed Mice With Hyperlipidemia Conditions
Erratum in
-
CaMKII Activation Promotes Cardiac Electrical Remodeling and Increases the Susceptibility to Arrhythmia Induction in High-fat Diet-Fed Mice With Hyperlipidemia Conditions: Erratum.J Cardiovasc Pharmacol. 2018 Jun;71(6):387. doi: 10.1097/FJC.0000000000000589. J Cardiovasc Pharmacol. 2018. PMID: 29864081 No abstract available.
Abstract
Background: Obesity/hyperlipidemia is closely related to both atrial and ventricular arrhythmias. CaMKII, a multifunctional serine/threonine kinase, has been involved in cardiac arrhythmias of different etiologies. However, its role in obesity/hyperlipidemia-related cardiac arrhythmia is unexplored. The aim of this was to determine the involvement of CaMKII in the process.
Methods: Adult male APOE mice were fed a high-fat diet (HFD), administrated with KN93 (10 mg·kg·2d), a specific inhibitor of CaMKII. Serum lipid and glucose profile, cardiac function, and surface electrocardiogram were determined. Electrophysiological study and epicardial activation mapping were performed in Langendorff-perfused heart. Expression of cardiac ion channels, gap junction proteins, Ca handling proteins, and CaMKII were evaluated, coupled with histological analysis.
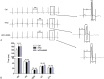
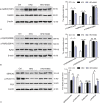
Results: A hyperlipidemia condition was induced by HFD in the APOE mice, which was associated with increased expression and activity of CaMKII in the hearts. In Langendorff-perfused hearts, HFD-induced heart showed increased arrhythmia inducibility, prolonged action potential duration, and decreased action potential duration alternans thresholds, coupled with slow ventricular conduction, connexin-43 upregulation, and interstitial fibrosis. Downregulation of ion channels including Cav1.2 and Kv4.2/Kv4.3 and disturbed Ca handling proteins were also observed in HFD-induced heart. Interestingly, all these alterations were significantly inhibited by KN93 treatment.
Conclusion: Our results demonstrated an adverse effect of metabolic components on cardiac electrophysiology and implicated an important role of CaMKII underlying this process.
Conflict of interest statement
The authors report no conflicts of interest.
Figures



Similar articles
-
Role of CaMKII in free fatty acid/hyperlipidemia-induced cardiac remodeling both in vitro and in vivo.J Mol Cell Cardiol. 2017 Aug;109:1-16. doi: 10.1016/j.yjmcc.2017.06.010. Epub 2017 Jun 28. J Mol Cell Cardiol. 2017. PMID: 28668302
-
Loss of MD1 increases vulnerability to ventricular arrhythmia in diet-induced obesity mice via enhanced activation of the TLR4/MyD88/CaMKII signaling pathway.Nutr Metab Cardiovasc Dis. 2019 Sep;29(9):991-998. doi: 10.1016/j.numecd.2019.06.004. Epub 2019 Jun 20. Nutr Metab Cardiovasc Dis. 2019. PMID: 31353205
-
CaMKII-dependent late Na+ current increases electrical dispersion and arrhythmia in ischemia-reperfusion.Am J Physiol Heart Circ Physiol. 2018 Oct 1;315(4):H794-H801. doi: 10.1152/ajpheart.00197.2018. Epub 2018 Jun 22. Am J Physiol Heart Circ Physiol. 2018. PMID: 29932771 Free PMC article.
-
Cardiac calmodulin kinase: a potential target for drug design.Curr Med Chem. 2011;18(24):3707-13. doi: 10.2174/092986711796642409. Curr Med Chem. 2011. PMID: 21774758 Free PMC article. Review.
-
Atrial remodelling in atrial fibrillation: CaMKII as a nodal proarrhythmic signal.Cardiovasc Res. 2016 Apr 1;109(4):542-57. doi: 10.1093/cvr/cvw002. Epub 2016 Jan 13. Cardiovasc Res. 2016. PMID: 26762270 Free PMC article. Review.
Cited by
-
New Insights on the Role of Connexins and Gap Junctions Channels in Adipose Tissue and Obesity.Int J Mol Sci. 2021 Nov 10;22(22):12145. doi: 10.3390/ijms222212145. Int J Mol Sci. 2021. PMID: 34830025 Free PMC article. Review.
-
Study on Toll-Like Receptor 2-Mediated Inflammation-Induced Familial Hypertension Combined with Hyperlipemia and Its Mechanism.J Healthc Eng. 2022 Jan 5;2022:1473597. doi: 10.1155/2022/1473597. eCollection 2022. J Healthc Eng. 2022. Retraction in: J Healthc Eng. 2023 Feb 18;2023:9860913. doi: 10.1155/2023/9860913. PMID: 35035808 Free PMC article. Retracted.
-
Impact of Obesity on Atrial Fibrillation Pathogenesis and Treatment Options.J Am Heart Assoc. 2024 Jan 2;13(1):e032277. doi: 10.1161/JAHA.123.032277. Epub 2023 Dec 29. J Am Heart Assoc. 2024. PMID: 38156451 Free PMC article. Review.
-
Advanced glycation end products modulate electrophysiological remodeling of right ventricular outflow tract cardiomyocytes: A novel target for diabetes-related ventricular arrhythmogenesis.Physiol Rep. 2022 Nov;10(21):e15499. doi: 10.14814/phy2.15499. Physiol Rep. 2022. PMID: 36325589 Free PMC article.
-
Diabetes and Arrhythmias: Pathophysiology, Mechanisms and Therapeutic Outcomes.Front Physiol. 2018 Nov 26;9:1669. doi: 10.3389/fphys.2018.01669. eCollection 2018. Front Physiol. 2018. PMID: 30534081 Free PMC article. Review.
References
-
- Tsang TS, Petty GW, Barnes ME, et al. The prevalence of atrial fibrillation in incident stroke cases and matched population controls in Rochester, Minnesota: changes over three decades. J Am Coll Cardiol. 2003;42:93–100. - PubMed
-
- Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. JAMA. 2004;292:2471–2477. - PubMed
-
- Aubin M-C, Cardin S, Comtois P, et al. A high-fat diet increases risk of ventricular arrhythmia in female rats: enhanced arrhythmic risk in the absence of obesity or hyperlipidemia. J Appl Physiol. 2010;108:933–940. - PubMed
-
- Savio-Galimberti E, Kannankeril P, Wasserman D, et al. Weight loss reduces atrial fibrillation inducibility and burden in severe obesity induced by either high-fat diet or genetic hyperphagia in mice. Circulation. 2015;132(suppl 3):A14729–A.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

