Update: Influenza Activity in the United States During the 2016-17 Season and Composition of the 2017-18 Influenza Vaccine
- PMID: 28662019
- PMCID: PMC5687497
- DOI: 10.15585/mmwr.mm6625a3
Update: Influenza Activity in the United States During the 2016-17 Season and Composition of the 2017-18 Influenza Vaccine
Abstract
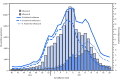
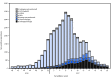
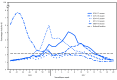
During the 2016-17 influenza season (October 2, 2016-May 20, 2017) in the United States, influenza activity* was moderate. Activity remained low through November, increased during December, and peaked in February nationally, although there were regional differences in the timing of influenza activity. Influenza A(H3N2) viruses predominated through mid-March and were predominant overall for the season, but influenza B viruses were most commonly reported from late March through May. This report summarizes influenza activity in the United States during October 2, 2016-May 20, 2017† and updates the previous summary (1).
Conflict of interest statement
Figures
Similar articles
-
Update: Influenza Activity - United States, October 2, 2016-February 4, 2017.MMWR Morb Mortal Wkly Rep. 2017 Feb 17;66(6):159-166. doi: 10.15585/mmwr.mm6606a2. MMWR Morb Mortal Wkly Rep. 2017. PMID: 28207684 Free PMC article.
-
Update: Influenza Activity - United States, October 1-November 25, 2017.MMWR Morb Mortal Wkly Rep. 2017 Dec 8;66(48):1318-1326. doi: 10.15585/mmwr.mm6648a2. MMWR Morb Mortal Wkly Rep. 2017. PMID: 29216030 Free PMC article.
-
Influenza Activity - United States, 2015-16 Season and Composition of the 2016-17 Influenza Vaccine.MMWR Morb Mortal Wkly Rep. 2016 Jun 10;65(22):567-75. doi: 10.15585/mmwr.mm6522a3. MMWR Morb Mortal Wkly Rep. 2016. PMID: 27281364
-
Update: Influenza Activity in the United States During the 2017-18 Season and Composition of the 2018-19 Influenza Vaccine.MMWR Morb Mortal Wkly Rep. 2018 Jun 8;67(22):634-642. doi: 10.15585/mmwr.mm6722a4. MMWR Morb Mortal Wkly Rep. 2018. PMID: 29879098 Free PMC article.
-
Update: Influenza Activity - United States, October 2-December 17, 2016.MMWR Morb Mortal Wkly Rep. 2016 Dec 30;65(50-51):1439-1444. doi: 10.15585/mmwr.mm655051a5. MMWR Morb Mortal Wkly Rep. 2016. PMID: 28033315
Cited by
-
Detection of Victoria lineage influenza B viruses with K162 and N163 deletions in the hemagglutinin gene, South Africa, 2018.Health Sci Rep. 2021 Sep 17;4(3):e367. doi: 10.1002/hsr2.367. eCollection 2021 Sep. Health Sci Rep. 2021. PMID: 34557595 Free PMC article.
-
Pharmacokinetics of the Monoclonal Antibody MHAA4549A Administered in Combination With Oseltamivir in Patients Hospitalized With Severe Influenza A Infection.J Clin Pharmacol. 2020 Nov;60(11):1509-1518. doi: 10.1002/jcph.1652. Epub 2020 Jul 4. J Clin Pharmacol. 2020. PMID: 32621543 Free PMC article. Clinical Trial.
-
IRF7-deficient MDCK cell based on CRISPR/Cas9 technology for enhancing influenza virus replication and improving vaccine production.PeerJ. 2022 Sep 21;10:e13989. doi: 10.7717/peerj.13989. eCollection 2022. PeerJ. 2022. PMID: 36164603 Free PMC article.
-
A Dose-finding Study of a Wild-type Influenza A(H3N2) Virus in a Healthy Volunteer Human Challenge Model.Clin Infect Dis. 2019 Nov 27;69(12):2082-2090. doi: 10.1093/cid/ciz141. Clin Infect Dis. 2019. PMID: 30770534 Free PMC article.
-
[Efficacy, effectiveness and safety of the adjuvanted influenza vaccine in the population aged 65 or over].Rev Esp Quimioter. 2023 Aug;36(4):334-345. doi: 10.37201/req/145.2022. Epub 2023 Apr 20. Rev Esp Quimioter. 2023. PMID: 37079707 Free PMC article. Review. Spanish.
References
-
- Food and Drug Administration. Summary minutes: meeting of the Vaccines and Related Biological Products Advisory Committee. Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2017. https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMateri...
-
- CDC. Disease burden of influenza. Atlanta, GA: US Department of Health and Human Services, CDC; 2017. https://www.cdc.gov/flu/about/disease/burden.htm
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

