Activation of complement factor B contributes to murine and human myocardial ischemia/reperfusion injury
- PMID: 28662037
- PMCID: PMC5491012
- DOI: 10.1371/journal.pone.0179450
Activation of complement factor B contributes to murine and human myocardial ischemia/reperfusion injury
Abstract
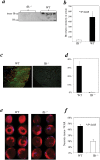
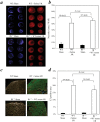
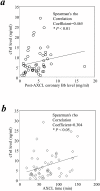
The pathophysiology of myocardial injury that results from cardiac ischemia and reperfusion (I/R) is incompletely understood. Experimental evidence from murine models indicates that innate immune mechanisms including complement activation via the classical and lectin pathways are crucial. Whether factor B (fB), a component of the alternative complement pathway required for amplification of complement cascade activation, participates in the pathophysiology of myocardial I/R injury has not been addressed. We induced regional myocardial I/R injury by transient coronary ligation in WT C57BL/6 mice, a manipulation that resulted in marked myocardial necrosis associated with activation of fB protein and myocardial deposition of C3 activation products. In contrast, in fB-/- mice, the same procedure resulted in significantly reduced myocardial necrosis (% ventricular tissue necrotic; fB-/- mice, 20 ± 4%; WT mice, 45 ± 3%; P < 0.05) and diminished deposition of C3 activation products in the myocardial tissue (fB-/- mice, 0 ± 0%; WT mice, 31 ± 6%; P<0.05). Reconstitution of fB-/- mice with WT serum followed by cardiac I/R restored the myocardial necrosis and activated C3 deposition in the myocardium. In translational human studies we measured levels of activated fB (Bb) in intracoronary blood samples obtained during cardio-pulmonary bypass surgery before and after aortic cross clamping (AXCL), during which global heart ischemia was induced. Intracoronary Bb increased immediately after AXCL, and the levels were directly correlated with peripheral blood levels of cardiac troponin I, an established biomarker of myocardial necrosis (Spearman coefficient = 0.465, P < 0.01). Taken together, our results support the conclusion that circulating fB is a crucial pathophysiological amplifier of I/R-induced, complement-dependent myocardial necrosis and identify fB as a potential therapeutic target for prevention of human myocardial I/R injury.
Conflict of interest statement
Figures


References
-
- Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nature medicine. 2011;17(11):1391–401. Epub 2011/11/09. doi: 10.1038/nm.2507 ;. - DOI - PMC - PubMed
-
- Millington TM, Madsen JC. Innate immunity and cardiac allograft rejection. Kidney Int Suppl. 2010;(119):S18–21. Epub 2010/12/01. doi: 10.1038/ki.2010.417 ;. - DOI - PMC - PubMed
-
- Lee JC, Christie JD. Primary graft dysfunction. Clin Chest Med. 2011;32(2):279–93. Epub 2011/04/23. doi: 10.1016/j.ccm.2011.02.007 . - DOI - PubMed
-
- Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117(16):2131–41. Epub 2008/04/23. doi: 10.1161/CIRCULATIONAHA.107.711911 . - DOI - PubMed
-
- Arcasoy SM, Fisher A, Hachem RR, Scavuzzo M, Ware LB. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction part V: predictors and outcomes. The Journal of heart and lung transplantation: the official publication of the International Society for Heart Transplantation. 2005;24(10):1483–8. Epub 2005/10/08. doi: 10.1016/j.healun.2004.11.314 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

