Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms
- PMID: 28662202
- PMCID: PMC5491144
- DOI: 10.1371/journal.pone.0180183
Innovative non-thermal plasma disinfection process inside sealed bags: Assessment of bactericidal and sporicidal effectiveness in regard to current sterilization norms
Abstract
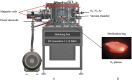
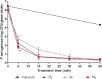
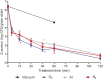
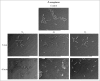
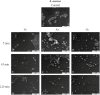
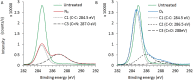
In this work, we developed a device capable to generate a non-thermal plasma discharge inside a sealed bag. The aim of this study was to assess the effectiveness of the oxygen, nitrogen and argon plasma sterilization on Pseudomonas aeruginosa, Staphylococcus aureus and Bacillus subtilis spores according to the NF EN 556 Norm. Moreover the bag integrity which is a critical key to maintain the sterile state of items after the end of the process was verified by Fourier Transform Infrared (FTIR) and X-ray Photoelectron Spectrometry (XPS) analyses. After plasma treatments, the bacterial counting showed a 6 log reduction of P. aeruginosa and S. aureus in 45 min and 120 min respectively whatever the gas used and a 4 log reduction of B. subtilis spores in 120 min with only oxygen plasma. These results were confirmed by Scanning Electron Microscopy (SEM) observations showing altered bacteria or spores and numerous debris. Taking into account the studied microorganisms, the oxygen plasma treatment showed the highest efficiency. FTIR and XPS analyses showed that this treatment induced no significant modification of the bags. To conclude this non-thermal plasma sterilization technique could be an opportunity to sterilize heat and chemical-sensitive medical devices and to preserve their sterile state after the end of the process.
Conflict of interest statement
Figures




References
-
- Moreau M, Orange N, Feuilloley MGJ, Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008; 26: 610–617. doi: 10.1016/j.biotechadv.2008.08.001 - DOI - PubMed
-
- Steelman VM, Ethylene oxide. The importance of aeration. AORN J. 1995; 55: 773–5, 778–9, 782–783. - PubMed
-
- Holyoak GR, Wang S, Liu Y, Bunch TD, Toxic effects of ethylene oxide residues on bovine embryos in vitro. Toxicology 1996; 108: 33–38. - PubMed
-
- Zhang YZ, Bjursten LM, Freij-Larsson C, Kober M, Wesslén B, Tissue response to commercial silicone and polyurethane elastomers after different sterilization procedures. Biomaterials. 1996; 17: 2265–2272. - PubMed
-
- Lerouge S, Wertheimer MR, Yahia L, Plasma Sterilization: A Review of Parameters, Mechanisms, and Limitations. Plasmas and Polym. 2001; 6: 175–188.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

