Protease-Cleavable Linkers Modulate the Anticancer Activity of Noninternalizing Antibody-Drug Conjugates
- PMID: 28662334
- PMCID: PMC5521252
- DOI: 10.1021/acs.bioconjchem.7b00304
Protease-Cleavable Linkers Modulate the Anticancer Activity of Noninternalizing Antibody-Drug Conjugates
Abstract
Antibody-drug conjugates (ADCs) represent an attractive class of biopharmaceutical agents, with the potential to selectively deliver potent cytotoxic agents to tumors. It is generally assumed that ADC products should preferably bind and internalize into cancer cells in order to liberate their toxic payload, but a growing body of evidence indicates that also ADCs based on noninternalizing antibodies may be potently active. In this Communication, we investigated dipeptide-based linkers (frequently used for internalizing ADC products) in the context of the noninternalizing F16 antibody, specific to a splice isoform of tenascin-C. Using monomethyl auristatin E (MMAE) as potent cytotoxic drug, we observed that a single amino acid substitution of the Val-Cit dipeptide linker can substantially modulate the in vivo stability of the corresponding ADC products, as well as the anticancer activity in mice bearing the human epidermoid A431 carcinoma. In these settings, the linker based on the Val-Ala dipeptide exhibited better performances, compared to Val-Cit, Val-Lys, and Val-Arg analogues. Mass spectrometric analysis revealed that the four linkers displayed not only different stability in vivo but also differences in cleavage sites. Moreover, the absence of anticancer activity for a F16-MMAE conjugate featuring a noncleavable linker indicated that drug release modalities, based on proteolytic degradation of the immunoglobulin moiety, cannot be exploited with noninternalizing antibodies. ADC products based on the noninternalizing F16 antibody may be useful for the treatment of several human malignancies, as the cognate antigen is abundantly expressed in the extracellular matrix of several tumors, while being virtually undetectable in most normal adult tissues.
Figures
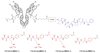

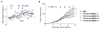
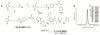

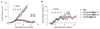

Similar articles
-
Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix.Int J Cancer. 2017 Apr 1;140(7):1670-1679. doi: 10.1002/ijc.30569. Epub 2016 Dec 30. Int J Cancer. 2017. PMID: 27943268 Free PMC article.
-
Development and Properties of Valine-Alanine based Antibody-Drug Conjugates with Monomethyl Auristatin E as the Potent Payload.Int J Mol Sci. 2017 Aug 25;18(9):1860. doi: 10.3390/ijms18091860. Int J Mol Sci. 2017. PMID: 28841157 Free PMC article.
-
Optimizing Lysosomal Activation of Antibody-Drug Conjugates (ADCs) by Incorporation of Novel Cleavable Dipeptide Linkers.Mol Pharm. 2019 Dec 2;16(12):4817-4825. doi: 10.1021/acs.molpharmaceut.9b00696. Epub 2019 Oct 29. Mol Pharm. 2019. PMID: 31609629
-
Antibody-Drug Conjugate Payloads; Study of Auristatin Derivatives.Chem Pharm Bull (Tokyo). 2020;68(3):201-211. doi: 10.1248/cpb.c19-00853. Chem Pharm Bull (Tokyo). 2020. PMID: 32115527 Review.
-
Cleavable linkers in antibody-drug conjugates.Chem Soc Rev. 2019 Aug 12;48(16):4361-4374. doi: 10.1039/c8cs00676h. Chem Soc Rev. 2019. PMID: 31294429 Review.
Cited by
-
Glutamic acid-valine-citrulline linkers ensure stability and efficacy of antibody-drug conjugates in mice.Nat Commun. 2018 Jun 28;9(1):2512. doi: 10.1038/s41467-018-04982-3. Nat Commun. 2018. PMID: 29955061 Free PMC article.
-
Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required?Br J Cancer. 2017 Dec 5;117(12):1736-1742. doi: 10.1038/bjc.2017.367. Epub 2017 Oct 24. Br J Cancer. 2017. PMID: 29065110 Free PMC article. Review.
-
Multivalency Increases the Binding Strength of RGD Peptidomimetic-Paclitaxel Conjugates to Integrin αV β3.Chemistry. 2017 Oct 17;23(58):14410-14415. doi: 10.1002/chem.201703093. Epub 2017 Sep 6. Chemistry. 2017. PMID: 28816404 Free PMC article.
-
Peptide-Drug Conjugates: A New Hope for Cancer.J Pept Sci. 2025 Aug;31(8):e70040. doi: 10.1002/psc.70040. J Pept Sci. 2025. PMID: 40646707 Free PMC article. Review.
-
Enhanced Therapeutic Activity of Non-Internalizing Small-Molecule-Drug Conjugates Targeting Carbonic Anhydrase IX in Combination with Targeted Interleukin-2.Clin Cancer Res. 2018 Aug 1;24(15):3656-3667. doi: 10.1158/1078-0432.CCR-17-3457. Epub 2018 Apr 24. Clin Cancer Res. 2018. PMID: 29691298 Free PMC article.
References
-
- Bosslet K, Straub R, Blumrich M, Czech J, Gerken M, Sperker B, Kroemer HK, Gesson JP, Koch M, Monneret C. Elucidation of the mechanism enabling tumor selective prodrug monotherapy. Cancer Res. 1998;58:1195–1201. - PubMed
-
- Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Curr Opin Chem Biol. 2010;14:529–537. - PubMed
-
- Gerber H-P, Koehnb FE, Abraham RT. The antibody-drug conjugate: an enabling modality for natural product-based cancer therapeutics. Nat Prod Rep. 2013;30:625–639. - PubMed
-
- Chu Y-W, Polson A. Antibody-drug conjugates for the treatment of B-cell non-Hodgkin's lymphoma and leukemia. Future Oncol. 2013;9:355–368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

