An automated synthesizer for electrochemical 18F-fluorination of organic compounds
- PMID: 28662441
- PMCID: PMC5585111
- DOI: 10.1016/j.apradiso.2017.06.028
An automated synthesizer for electrochemical 18F-fluorination of organic compounds
Abstract
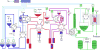
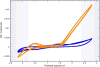
Electrochemical 18F-fluorination of organic compounds provides a means to synthesize Positron-Emission-Tomography (PET) tracers difficult to obtain otherwise. Here, the first automated synthesizer that enables radiolabeling through carrier-added electrochemical 18F-fluorination is described. The system provides capabilities for all necessary operations such as drying of cyclotron derived [18F]fluoride, electrochemical incorporation of the radioisotope into a precursor molecule, subsequent reactions such as protecting group removals, HPLC-purification and formulation of the final tracer. Demonstrated is the aliphatic electrochemical 18F-fluorination of methyl 2-(phenylthio)acetate.
Keywords: Electrochemistry; Late-stage fluorination; PET; Radiosynthesizer; [(18)F]fluoride.
Copyright © 2017. Published by Elsevier Ltd.
Figures





References
-
- Fuchigami T, Konno A, Nakagawa K, Shimojo M. Electrolytic partial fluorination of organic compounds. 12. Selective anodic monofluorination of fluoroalkyl and alkyl sulfides. J Org Chem. 1994;59:5937–5941.
-
- Fuchigami T, Inagi S. Selective electrochemical fluorination of organic molecules and macromolecules in ionic liquids. Chem Commun. 2011;47:10211–10223. - PubMed
-
- Fuchigami T, Kayoko Y, Akinori K. Electrolytic reactions of fluoro organic compounds. 8. Further study on anodic methoxylation and acetoxylation of aryl fluoroalkyl sulfides. Tetrahedron. 1991;47:625–634.
-
- Gao Z, et al. Metal-free oxidative fluorination of phenols with [18F]fluoride. Angew Chem Int Ed. 2012;51:6733–6737. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

