Systematic review of patient reported quality of life following stereotactic ablative radiotherapy for primary and metastatic liver cancer
- PMID: 28662680
- PMCID: PMC5492951
- DOI: 10.1186/s13014-017-0818-8
Systematic review of patient reported quality of life following stereotactic ablative radiotherapy for primary and metastatic liver cancer
Abstract
Background: Stereotactic ablative radiotherapy (SABR) is a safe and effective modality in patients with liver cancer who are ineligible for other local therapies. However SABR is not current standard of practice and requires further validation. Patient reported quality of life (QOL) is key to this validation, yet no systematic reviews to date have been performed to analyse QOL following liver SABR. QOL is a critical part of therapy evaluation, particularly in disease states with short life expectancy. The purpose of this study was to conduct a systematic review of QOL outcomes for liver SABR.
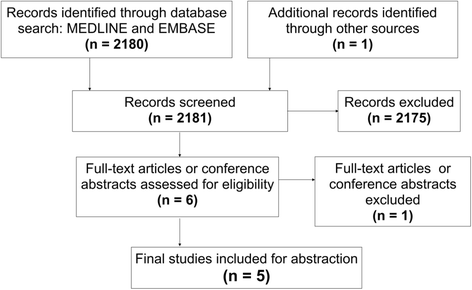
Materials and methods: MEDLINE and EMBASE databases from 1996 to October 2015 were queried to obtain English language studies analysing QOL following liver SABR. Included studies described patient-reported QOL as either a primary or secondary endpoint, and analysed QOL change over time. Studies were screened, and relevant data were abstracted and analysed.
Results: Of 2181 initially screened studies, 5 met all inclusion criteria. Extracted studies included a total of 392 eligible patients with hepatocellular carcinoma, liver metastases and intrahepatic cholangiocarcinoma. Four studies were prospective in design, and only one study was a conference abstract. Extracted studies were heterogeneous in dose prescription used (11-70 Gy in 3-30 fractions), in addition to reported QOL metrics (EORTC QLQ C-15 PAL,/C-30/LM-21, EuroQol 5D, FACT-Hep, FLIC) and final endpoints (range 6 weeks to 12 months). Despite this there were few statistically significant declines in QOL scores following SABR. Four studies demonstrated transient fatigue in the first 1-4 weeks, while 2 studies showed transient worsening of appetite at 1 month. In all but one instance (loss of appetite at 6 weeks), levels returned to insignificant difference baseline by the final endpoints. All studies showed no significant QOL decline in any domain at their respective endpoints. In studies with overlapping QOL tools, estimates of 3-month post SABR global QOL were similar.
Conclusion: Results of this systematic review demonstrate well-preserved post liver SABR QOL. These findings strengthen the argument for liver SABR, and should aim to support future comparative effectiveness trials with other local modalities including surgery, chemoembolization and radiofrequency ablation, with a focus on QOL outcomes as an important endpoint.
Keywords: Hepatocellular carcinoma; Liver metastases; QOL; Quality of life; SABR; SBRT; Stereotactic; Systematic review.
References
-
- Sahgal A, Roberge D, Schellenberg D, Purdie T, Swaminath A, Pantarotto J, Filion E, Gabos Z, Butler J, Letourneau D, Masucci G, Mulroy L, Bezjak A, Dawson L, Parliament M. The Canadian association of radiation oncology scope of practice guidelines for lung, liver and spine stereotactic body radiotherapy. Clin Oncol. 2012;24:629–639. doi: 10.1016/j.clon.2012.04.006. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous