Thrombocytopenia induced by dabigatran: two case reports
- PMID: 28662704
- PMCID: PMC5492117
- DOI: 10.1186/s12883-017-0900-8
Thrombocytopenia induced by dabigatran: two case reports
Abstract
Background: Vitamin K inhibitors (e.g. warfarin) and indirect thrombin inhibitors (e.g. heparin) are widely used to prevent thromboembolic disorders (e.g. myocardial infarction, venous thromboembolism, and stroke). These agents have been mainstays of anticoagulation for people older than 60 years. However, their administration is associated with a risk of bleeding and requires careful monitoring of patients. Novel oral anticoagulants (NOACs), such as dabigatran, are significantly safer in preventing thromboembolism than warfarin and heparin (sporadically causes thrombocytopenia) and are more specific for their target protein, thrombin. The major advantage of dabigatran, a direct thrombin inhibitor, is that it reversibly inhibits both free and clot-bound thrombin by tight binding affinity and the predictable pharmacodynamic effect. A few studies, however, reported that dabigatran can cause thrombocytopenia, although the underlying mechanism remains unclear. Thus, an antidote for dabigatran was developed to prevent thrombocytopenia.
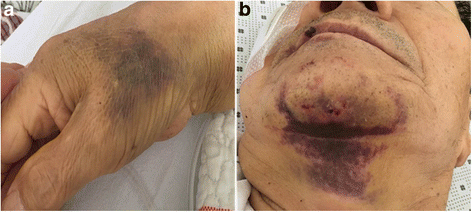
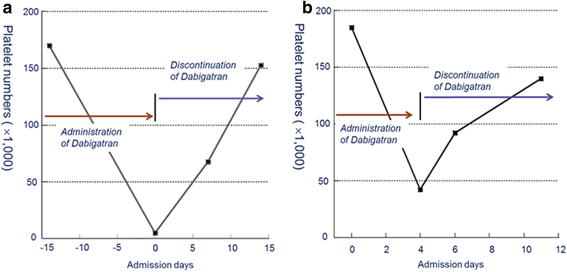
Case presentation: In this report, we discuss two cases of thrombocytopenia and purpura after dabigatran treatment. A 73-year-old man showed hemorrhagic necrotic skin lesions on his neck and right hand. He was administered dabigatran (220 mg/day) for cerebral infarction for three days and his platelet count decreased abruptly (6000/μL). This suggested that dabigatran had caused thrombocytopenia and purpura; therefore, dabigatran administration was discontinued. The results of a blood test, performed 14 days after stopping dabigatran treatment, showed that the platelet count had recovered to the normal range of more than 150,000/μL. A 75-year-old woman had taken warfarin continuously for 8 years. However, she had a new cerebral infarction. Therefore, warfarin treatment was replaced with dabigatran (300 mg/day). Her platelet count decreased (41,000/μL) significantly and dabigatran treatment was discontinued. The blood test results show that platelet counts gradually recovered to the normal range.
Conclusions: Dabigatran application may cause bleeding; therefore, careful monitoring during dabigatran treatment is required to prevent thrombocytopenia. An explanation is that the interaction of dabigatran with thrombin, because of its strong binding affinity, may cause the observed thrombocytopenia.
Keywords: Dabigatran; Factor Xa inhibitor; Thrombin inhibitor; Thrombocytopenia.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable for this case report.
Consent for publication
Written informed consents were obtained from the patients for publication of these Case Report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Riva N, Lip GYH. A new era for anticoagulation in atrial fibrillation which anticoagulant should we choose for long-term prevention of thromboembolic complications in patients with atrial fibrillation? Pol Arch Med Wewn. 2012;122:45–52. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

