Personal Protective Equipment in Animal Research
- PMID: 28662749
- PMCID: PMC5482512
Personal Protective Equipment in Animal Research
Abstract
The occupational health and safety program is an integral component of a comprehensive animal care and use program. It is important to mitigate the risk of exposures of animal care and research personnel to allergens and physical, chemical, radiologic, and biologic hazards during the conduct of various tasks. This need is especially true in infectious disease and biocontainment research. One aspect of the program is the provision of personal protective equipment (PPE). Commercially available PPE should be carefully evaluated based on their material composition and performance according to manufacturer data. To help institutions and end users by providing them guidance on choosing appropriate PPE, we here discuss the regulatory framework, device standards, and materials engineering for various PPE, including gloves, shoe covers, head caps, gowns, aprons, masks, hearing and eye protection devices, and respirators. Ultimately, the choice of appropriate PPE is based on the risk assessment, which should include consideration for personnel comfort, correct device fitting, and the containment level for the hazard used.
Figures
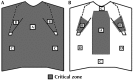
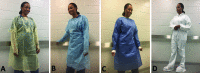


References
-
- American Latex Allergy Association. [Internet]. 1996. Definition. [Cited 14 January 2017] Available at http://latexallergyresources.org/definition.
-
- Association for the Advancement of Medical Instrumentation. 2005. TIR11:2005 Selection and use of protective apparel and surgical drapes in health care facilities. Arlington (VA): Association for the Advancement of Medical Instrumentation.
-
- Association for the Advancement of Medical Instrumentation. 2012. ANSI/AAMI PB70:2012 Liquid barrier performance and classification of protective apparel and drapes intended for use in health care facilities. Arlington (VA): Association for the Advancement of Medical Instrumentation.
-
- Association of the Nonwoven Fabrics Industry. [Internet]. 2012. Spunbond and melt–blown technology. [Cited 20 October 2016] Available at: http://www.inda.org/spunbond-melt-blown.html.
-
- Bardorf MH, Jäger B, Boeckmans E, Kramer A, Assadian O. 2016. Influence of material properties on gloves’ bacterial barrier efficacy in the presence of microperforation. Am J Infect Control 44: 1645–1649. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
