Decreased Axon Caliber Underlies Loss of Fiber Tract Integrity, Disproportional Reductions in White Matter Volume, and Microcephaly in Angelman Syndrome Model Mice
- PMID: 28663201
- PMCID: PMC5546107
- DOI: 10.1523/JNEUROSCI.0037-17.2017
Decreased Axon Caliber Underlies Loss of Fiber Tract Integrity, Disproportional Reductions in White Matter Volume, and Microcephaly in Angelman Syndrome Model Mice
Abstract
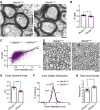
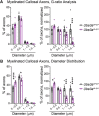
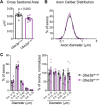
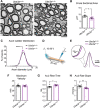
Angelman syndrome (AS) is a debilitating neurodevelopmental disorder caused by loss of function of the maternally inherited UBE3A allele. It is currently unclear how the consequences of this genetic insult unfold to impair neurodevelopment. We reasoned that by elucidating the basis of microcephaly in AS, a highly penetrant syndromic feature with early postnatal onset, we would gain new insights into the mechanisms by which maternal UBE3A loss derails neurotypical brain growth and function. Detailed anatomical analysis of both male and female maternal Ube3a-null mice reveals that microcephaly in the AS mouse model is primarily driven by deficits in the growth of white matter tracts, which by adulthood are characterized by densely packed axons of disproportionately small caliber. Our results implicate impaired axon growth in the pathogenesis of AS and identify noninvasive structural neuroimaging as a potentially valuable tool for gauging therapeutic efficacy in the disorder.SIGNIFICANCE STATEMENT People who maternally inherit a deletion or nonfunctional copy of the UBE3A gene develop Angelman syndrome (AS), a severe neurodevelopmental disorder. To better understand how loss of maternal UBE3A function derails brain development, we analyzed brain structure in a maternal Ube3a knock-out mouse model of AS. We report that the volume of white matter (WM) is disproportionately reduced in AS mice, indicating that deficits in WM development are a major factor underlying impaired brain growth and microcephaly in the disorder. Notably, we find that axons within the WM pathways of AS model mice are abnormally small in caliber. This defect is associated with slowed nerve conduction, which could contribute to behavioral deficits in AS, including motor dysfunction.
Keywords: Angelman; E6-AP; UBE3A; axon; microcephaly; white matter.
Copyright © 2017 the authors 0270-6474/17/377347-15$15.00/0.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
