Is It Possible to Develop a "Universal" Influenza Virus Vaccine? Immunogenetic Considerations Underlying B-Cell Biology in the Development of a Pan-Subtype Influenza A Vaccine Targeting the Hemagglutinin Stem
- PMID: 28663207
- PMCID: PMC6028068
- DOI: 10.1101/cshperspect.a029413
Is It Possible to Develop a "Universal" Influenza Virus Vaccine? Immunogenetic Considerations Underlying B-Cell Biology in the Development of a Pan-Subtype Influenza A Vaccine Targeting the Hemagglutinin Stem
Abstract
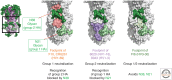
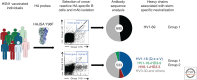
Current influenza vaccines preferentially generate B-cell responses to the variable hemagglutinin (HA) head. Focusing vaccine-induced antibody responses on epitopes in the conserved HA stem may provide better protection against future drifted and pandemic strains. Understanding the basis for the dominant HA head and subdominant HA stem-specific responses at the level of B-cell activation and differentiation will be critical for designing vaccines that induce sustained stem-specific responses. Identifying antibody lineages with broad neutralizing activity against influenza A viruses and defining the structural mode of recognition for germline precursors of those antibodies will also guide future immunogen design.
Copyright © 2018 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
