Testicular vs adrenal sources of hydroxy-androgens in prostate cancer
- PMID: 28663228
- PMCID: PMC5593253
- DOI: 10.1530/ERC-17-0107
Testicular vs adrenal sources of hydroxy-androgens in prostate cancer
Abstract
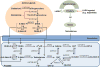
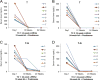
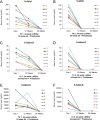
Neoadjuvant androgen deprivation therapy (NADT) is one strategy for the treatment of early-stage prostate cancer; however, the long-term outcomes of NADT with radical prostatectomy including biochemical failure-free survival are not promising. One proposed mechanism is incomplete androgen ablation. In this study, we aimed to evaluate the efficiency of serum hydroxy-androgen suppression in patients with localized high-risk prostate cancer under NADT (leuprolide acetate plus abiraterone acetate and prednisone) and interrogate the primary sources of circulating hydroxy-androgens using our recently described stable isotope dilution liquid chromatography mass spectrometric method. For the first time, three androgen diols including 5-androstene-3β,17β-diol (5-adiol), 5α-androstane-3α,17β-diol (3α-adiol), 5α-androstane-3β,17β-diol (3β-adiol), the glucuronide or sulfate conjugate of 5-adiol and 3α-adiol were measured and observed to be dramatically reduced after NADT. By comparing patients that took leuprolide acetate alone vs leuprolide acetate plus abiraterone acetate and prednisone, we were able to distinguish the primary sources of these androgens and their conjugates as being of either testicular or adrenal in origin. We find that testosterone, 5α-dihydrotestosterone (DHT), 3α-adiol and 3β-adiol were predominately of testicular origin. By contrast, dehydroepiandrosterone (DHEA), epi-androsterone (epi-AST) and their conjugates, 5-adiol sulfate and glucuronide were predominately of adrenal origin. Our findings also show that NADT failed to completely suppress DHEA-sulfate levels and that two unappreciated sources of intratumoral androgens that were not suppressed by leuprolide acetate alone were 5-adiol-sulfate and epi-AST-sulfate of adrenal origin.
Keywords: abiraterone acetate; androgen; localized high-risk prostate cancer; luteinizing hormone-releasing hormone agonist; stable isotope dilution liquid chromatography mass spectrometry.
© 2017 Society for Endocrinology.
Figures
Similar articles
-
Simultaneous quantitation of nine hydroxy-androgens and their conjugates in human serum by stable isotope dilution liquid chromatography electrospray ionization tandem mass spectrometry.J Steroid Biochem Mol Biol. 2017 Jan;165(Pt B):342-355. doi: 10.1016/j.jsbmb.2016.08.001. Epub 2016 Aug 12. J Steroid Biochem Mol Biol. 2017. PMID: 27531846 Free PMC article.
-
Androgen glucuronides analysis by liquid chromatography tandem-mass spectrometry: could it raise new perspectives in the diagnostic field of hormone-dependent malignancies?J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Dec 1;940:24-34. doi: 10.1016/j.jchromb.2013.09.022. Epub 2013 Sep 27. J Chromatogr B Analyt Technol Biomed Life Sci. 2013. PMID: 24140653 Review.
-
Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study.J Clin Oncol. 2014 Nov 20;32(33):3705-15. doi: 10.1200/JCO.2013.53.4578. Epub 2014 Oct 13. J Clin Oncol. 2014. PMID: 25311217 Free PMC article. Clinical Trial.
-
Development, validation and application of a stable isotope dilution liquid chromatography electrospray ionization/selected reaction monitoring/mass spectrometry (SID-LC/ESI/SRM/MS) method for quantification of keto-androgens in human serum.J Steroid Biochem Mol Biol. 2013 Nov;138:281-9. doi: 10.1016/j.jsbmb.2013.06.014. Epub 2013 Jul 10. J Steroid Biochem Mol Biol. 2013. PMID: 23851165 Free PMC article. Clinical Trial.
-
Assessment of steroidogenesis and steroidogenic enzyme functions.J Steroid Biochem Mol Biol. 2013 Sep;137:176-82. doi: 10.1016/j.jsbmb.2013.05.017. Epub 2013 Jun 13. J Steroid Biochem Mol Biol. 2013. PMID: 23770321 Review.
Cited by
-
Serum Androgen Metabolites Correlate with Clinical Variables in African and European American Men with Localized, Therapy Naïve Prostate Cancer.Metabolites. 2023 Feb 16;13(2):284. doi: 10.3390/metabo13020284. Metabolites. 2023. PMID: 36837903 Free PMC article.
-
Intracrinology-revisited and prostate cancer.J Steroid Biochem Mol Biol. 2020 Feb;196:105499. doi: 10.1016/j.jsbmb.2019.105499. Epub 2019 Oct 12. J Steroid Biochem Mol Biol. 2020. PMID: 31614208 Free PMC article. Review.
-
Dehydroepiandrosterone (DHEA)-SO4 Depot and Castration-Resistant Prostate Cancer.Vitam Horm. 2018;108:309-331. doi: 10.1016/bs.vh.2018.01.007. Epub 2018 Feb 24. Vitam Horm. 2018. PMID: 30029732 Free PMC article. Review.
-
Real-world analysis of leuprorelin acetate microspheres-based neoadjuvant therapy for patients with high-risk prostate cancer.Front Oncol. 2025 Mar 19;15:1520370. doi: 10.3389/fonc.2025.1520370. eCollection 2025. Front Oncol. 2025. PMID: 40177247 Free PMC article.
-
Cardiovascular toxicities associated with abiraterone compared to enzalutamide-A pharmacovigilance study.EClinicalMedicine. 2021 May 6;36:100887. doi: 10.1016/j.eclinm.2021.100887. eCollection 2021 Jun. EClinicalMedicine. 2021. PMID: 34308305 Free PMC article.
References
-
- Aus G, Abrahamsson PA, Ahlgren G, Hugosson J, Lundberg S, Schain M, Schelin S, Pedersen K. Three-month neoadjuvant hormonal therapy before radical prostatectomy: a 7-year follow-up of a randomized controlled trial. BJU International. 2002;90:561–566. doi: 10.1046/j.1464-410X.2002.02982.x. - DOI - PubMed
-
- Barbier O, Lapointe H, El Alfy M, Hum DW, Belanger A. Cellular localization of uridine diphosphoglucuronosyltransferase 2B enzymes in the human prostate by in situ hybridization and immunohistochemistry. Journal of Clinical Endocrinology and Metabolism. 2000;85:4819–4826. doi: 10.1210/jc.85.12.4819. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

