Complement Recognition Pathways in Renal Transplantation
- PMID: 28663231
- PMCID: PMC5576943
- DOI: 10.1681/ASN.2017010079
Complement Recognition Pathways in Renal Transplantation
Abstract
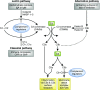
The complement system, consisting of soluble and cell membrane-bound components of the innate immune system, has defined roles in the pathophysiology of renal allograft rejection. Notably, the unavoidable ischemia-reperfusion injury inherent to transplantation is mediated through the terminal complement activation products C5a and C5b-9. Furthermore, biologically active fragments C3a and C5a, produced during complement activation, can modulate both antigen presentation and T cell priming, ultimately leading to allograft rejection. Earlier work identified renal tubule cell synthesis of C3, rather than hepatic synthesis of C3, as the primary source of C3 driving these effects. Recent efforts have focused on identifying the local triggers of complement activation. Collectin-11, a soluble C-type lectin expressed in renal tissue, has been implicated as an important trigger of complement activation in renal tissue. In particular, collectin-11 has been shown to engage L-fucose at sites of ischemic stress, activating the lectin complement pathway and directing the innate immune response to the distressed renal tubule. The interface between collectin-11 and L-fucose, in both the recipient and the allograft, is an attractive target for therapies intended to curtail renal inflammation in the acute phase.
Keywords: collectin-11; complement; lectin pathway; renal transplantation.
Copyright © 2017 by the American Society of Nephrology.
Figures


References
-
- Sacks SH, Zhou W: The role of complement in the early immune response to transplantation. Nat Rev Immunol 12: 431–442, 2012 - PubMed
-
- Pratt JR, Basheer SA, Sacks SH: Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med 8: 582–587, 2002 - PubMed
-
- Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, Suberbielle C, Frémeaux-Bacchi V, Méjean A, Desgrandchamps F, Anglicheau D, Nochy D, Charron D, Empana JP, Delahousse M, Legendre C, Glotz D, Hill GS, Zeevi A, Jouven X: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 - PubMed
-
- Marsh JE, Farmer CK, Jurcevic S, Wang Y, Carroll MC, Sacks SH: The allogeneic T and B cell response is strongly dependent on complement components C3 and C4. Transplantation 72: 1310–1318, 2001 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

