Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: a retrospective study of UK general practice
- PMID: 28663549
- PMCID: PMC5491501
- DOI: 10.1038/s41533-017-0040-z
Determinants of initial inhaled corticosteroid use in patients with GOLD A/B COPD: a retrospective study of UK general practice
Abstract
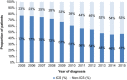
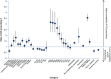
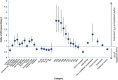
Initial use of inhaled corticosteroid therapy is common in patients with Global Initiative for Chronic Obstructive Lung Disease (GOLD) A or B chronic obstructive pulmonary disease, contrary to GOLD guidelines. We investigated UK prescribing of inhaled corticosteroid therapy in these patients, to identify predictors of inhaled corticosteroid use in newly diagnosed chronic obstructive pulmonary disease patients. A cohort of newly diagnosed GOLD A/B chronic obstructive pulmonary disease patients was identified from the UK Clinical Practice Research Datalink (June 2005-June 2015). Patients were classified by prescribed treatment, with those receiving inhaled corticosteroid-containing therapy compared with those receiving long-acting bronchodilators without inhaled corticosteroid. In all, 29,815 patients with spirometry-confirmed chronic obstructive pulmonary disease were identified. Of those prescribed maintenance therapy within 3 months of diagnosis, 63% were prescribed inhaled corticosteroid-containing therapy vs. 37% prescribed non-inhaled corticosteroid therapy. FEV1% predicted, concurrent asthma diagnosis, region, and moderate exacerbation were the strongest predictors of inhaled corticosteroid use in the overall cohort. When concurrent asthma patients were excluded, all other co-variates remained significant predictors. Other significant predictors included general practitioner practice, younger age, and co-prescription with short-acting bronchodilators. Trends over time showed that initial inhaled corticosteroid prescriptions reduced throughout the study, but still accounted for 47% of initial prescriptions in 2015. These results suggest that inhaled corticosteroid prescribing in GOLD A/B patients is common, with significant regional variation that is independent of FEV1% predicted.
Early-stage chronic lung disease: OVERUSE OF INHALED STEROIDS IN THE UK: Inhaled steroids are often prescribed to early-stage chronic lung disease patients in the UK despite guidelines to the contrary. Patients newly diagnosed with early-stage chronic obstructive pulmonary disease (COPD) should not be prescribed inhaled corticosteroids (ICS), because they carry an increased risk of side effects such as pneumonia and osteoporosis. ICS should be reserved for patients with severe COPD and frequent exacerbations. James Chalmers at the Scottish Centre for Respiratory Research, Dundee, and co-workers examined prescribed medication data from the UK spanning 10 years, to determine key predictors of ICS prescription during early-stage COPD. Of 29,815 patients identified, an average of 63% were prescribed ICS upon diagnosis, regardless of disease severity. Younger patients were more likely to receive ICS, possibly due to co-morbidity with chronic asthma, and particular UK regions and medical practices prescribed ICS more readily than others.
Conflict of interest statement
Competing interests
J.D.C. has received funding from the Wellcome Trust, Medical Research Council, Chief Scientist Office, Scottish Government, Tenovus Scotland, Bayer HealthCare, European Respiratory Society, AstraZeneca, Basilea, GlaxoSmithKline, Boehringer Ingelheim, Napp Pharmaceuticals, Pfizer and Chiesi. Ab.T., A.G., and An.T. are employees of Boehringer Ingelheim, the study sponsor. N.R. was an employee of Boehringer Ingelheim at the time of involvement in the study.
Ethical approval
Methods were performed in accordance with relevant regulations/guidelines. This study was reviewed and approved by the Independent Scientific Advisory Committee for Medicines and Healthcare products Regulatory Agency database research (Ref: 15_237 R) and an internal scientific committee within the study sponsor. As this was a non-interventional study using anonymised data, no patient consent was necessary.
Figures


References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD): Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (2016). - PubMed
-
- National Institute for Health and Care Excellence. Chronic obstructive pulmonary disease in over 16s: diagnosis and management. Clinical Guideline [CG101] (published 2010). https://www.nice.org.uk/guidance/cg101. Accessed March 2017. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

