Rare Variant Analysis of Human and Rodent Obesity Genes in Individuals with Severe Childhood Obesity
- PMID: 28663568
- PMCID: PMC5491520
- DOI: 10.1038/s41598-017-03054-8
Rare Variant Analysis of Human and Rodent Obesity Genes in Individuals with Severe Childhood Obesity
Abstract
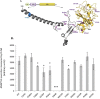
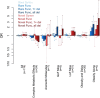
Obesity is a genetically heterogeneous disorder. Using targeted and whole-exome sequencing, we studied 32 human and 87 rodent obesity genes in 2,548 severely obese children and 1,117 controls. We identified 52 variants contributing to obesity in 2% of cases including multiple novel variants in GNAS, which were sometimes found with accelerated growth rather than short stature as described previously. Nominally significant associations were found for rare functional variants in BBS1, BBS9, GNAS, MKKS, CLOCK and ANGPTL6. The p.S284X variant in ANGPTL6 drives the association signal (rs201622589, MAF~0.1%, odds ratio = 10.13, p-value = 0.042) and results in complete loss of secretion in cells. Further analysis including additional case-control studies and population controls (N = 260,642) did not support association of this variant with obesity (odds ratio = 2.34, p-value = 2.59 × 10-3), highlighting the challenges of testing rare variant associations and the need for very large sample sizes. Further validation in cohorts with severe obesity and engineering the variants in model organisms will be needed to explore whether human variants in ANGPTL6 and other genes that lead to obesity when deleted in mice, do contribute to obesity. Such studies may yield druggable targets for weight loss therapies.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- MC_UU_12012/1/MRC_/Medical Research Council/United Kingdom
- G0800270/MRC_/Medical Research Council/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- R01 DK110403/DK/NIDDK NIH HHS/United States
- MR/P013880/1/MRC_/Medical Research Council/United Kingdom
- RG/08/014/24067/BHF_/British Heart Foundation/United Kingdom
- MC_UU_12012/5/MRC_/Medical Research Council/United Kingdom
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12015/1/MRC_/Medical Research Council/United Kingdom
- RG/15/14/31880/BHF_/British Heart Foundation/United Kingdom
- RG/16/4/32218/BHF_/British Heart Foundation/United Kingdom
- 268834/ERC_/European Research Council/International
- MR/P02811X/1/MRC_/Medical Research Council/United Kingdom
- R25 GM058903/GM/NIGMS NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- MR/L010305/1/MRC_/Medical Research Council/United Kingdom
- MC_UU_12013/8/MRC_/Medical Research Council/United Kingdom
- CH/12/2/29428/BHF_/British Heart Foundation/United Kingdom
- G0900554/MRC_/Medical Research Council/United Kingdom
- R01 DK064265/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

