Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water
- PMID: 28663825
- PMCID: PMC5480276
- DOI: 10.1016/j.jare.2017.06.002
Applications of CTAB modified magnetic nanoparticles for removal of chromium (VI) from contaminated water
Abstract
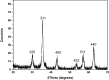
This study investigated the elimination of Cr(VI) from aqueous solution utilizing a composite from magnetic nanoparticles (Fe3O4) capped with cetyltrimethylammonium bromide (CTAB). The structure of the prepared composite system was examined by Fourier Transform Infra Red Spectroscopy (FTIR), X-ray Diffractometry (XRD), and Transmission Electron Microscopy (TEM). Separation of the Fe3O4/CTAB composite from the wastewater can be achieved by application of an external magnetic field. Factors affecting the Cr(VI) expulsion from wastewater such as pH, competing ions, the dosage level of the nanoparticles, and contact time were studied. The results indicated that the maximum efficiency of the present system for removal of Cr(VI) (95.77%) was in acidic conditions (pH 4), contact time 12 h, and composite dosage of 12 mg/mL. The used Cr(VI) concentration was 100 mg/L. Considering results, the Fe3O4/CTAB system showed a high capability and selectivity for the treatment of water sullied with Cr(VI). This can recede the mutagenic and carcinogenic health risk caused by Cr(VI) water tainting.
Keywords: Composite dosage; Cr(VI); Magnetic nanoparticles; TEM; XRD; pH.
Figures










References
-
- Kotas J., Stasicka Z. Chromium occurrence in the environment and methods of its speciation. Environ Pollut. 2000;107:263–283. - PubMed
-
- Zhao Y.-G., Shen H.-Y., Pan S.-D., Hu M.-Q., Xia Q.-H. Preparation and characterization of amino-functionalized nano-Fe3O4 magnetic polymer adsorbents for removal of chromium (VI) ions. J Mater Sci. 2010;45:5291–5301.
-
- Khan T.A., Nazir M., Ali I., Kumar A. Removal of chromium (VI) from aqueous solution using guar gum–nano zinc oxide biocomposite adsorbent. Arab J Chem. 2013;10(Suppl. 2):S2388–S2398.
-
- Burks T., Uheida A., Saleemi M., Eita M., Toprak M.S., Muhammed M. Removal of chromium (VI) using surface modified superparamagnetic iron oxide nanoparticles. Separ Sci Technol. 2013;48:1243–1251.
LinkOut - more resources
Full Text Sources
Other Literature Sources

