Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging [Invited]
- PMID: 28663890
- PMCID: PMC5480497
- DOI: 10.1364/BOE.8.002536
Review of adaptive optics OCT (AO-OCT): principles and applications for retinal imaging [Invited]
Abstract
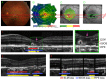
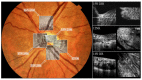
In vivo imaging of the human retina with a resolution that allows visualization of cellular structures has proven to be essential to broaden our knowledge about the physiology of this precious and very complex neural tissue that enables the first steps in vision. Many pathologic changes originate from functional and structural alterations on a cellular scale, long before any degradation in vision can be noted. Therefore, it is important to investigate these tissues with a sufficient level of detail in order to better understand associated disease development or the effects of therapeutic intervention. Optical retinal imaging modalities rely on the optical elements of the eye itself (mainly the cornea and lens) to produce retinal images and are therefore affected by the specific arrangement of these elements and possible imperfections in curvature. Thus, aberrations are introduced to the imaging light and image quality is degraded. To compensate for these aberrations, adaptive optics (AO), a technology initially developed in astronomy, has been utilized. However, the axial sectioning provided by retinal AO-based fundus cameras and scanning laser ophthalmoscope instruments is limited to tens of micrometers because of the rather small available numerical aperture of the eye. To overcome this limitation and thus achieve much higher axial sectioning in the order of 2-5µm, AO has been combined with optical coherence tomography (OCT) into AO-OCT. This enabled for the first time in vivo volumetric retinal imaging with high isotropic resolution. This article summarizes the technical aspects of AO-OCT and provides an overview on its various implementations and some of its clinical applications. In addition, latest developments in the field, such as computational AO-OCT and wavefront sensor less AO-OCT, are covered.
Keywords: (010.1080) Active or adaptive optics; (110.1758) Computational imaging; (170.2655) Functional monitoring and imaging; (170.4460) Ophthalmic optics and devices; (170.4500) Optical coherence tomography; (170.5755) Retina scanning; (330.5310) Vision - photoreceptors.
Figures
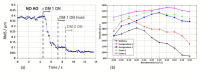
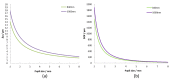

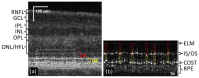


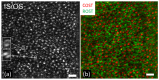





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
