Raman active components of skin cancer
- PMID: 28663910
- PMCID: PMC5480433
- DOI: 10.1364/BOE.8.002835
Raman active components of skin cancer
Abstract
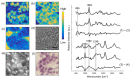
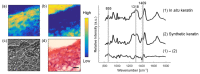
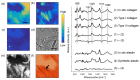
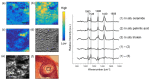
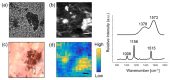
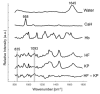
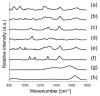
Raman spectroscopy (RS) has shown great potential in noninvasive cancer screening. Statistically based algorithms, such as principal component analysis, are commonly employed to provide tissue classification; however, they are difficult to relate to the chemical and morphological basis of the spectroscopic features and underlying disease. As a result, we propose the first Raman biophysical model applied to in vivo skin cancer screening data. We expand upon previous models by utilizing in situ skin constituents as the building blocks, and validate the model using previous clinical screening data collected from a Raman optical fiber probe. We built an 830nm confocal Raman microscope integrated with a confocal laser-scanning microscope. Raman imaging was performed on skin sections spanning various disease states, and multivariate curve resolution (MCR) analysis was used to resolve the Raman spectra of individual in situ skin constituents. The basis spectra of the most relevant skin constituents were combined linearly to fit in vivo human skin spectra. Our results suggest collagen, elastin, keratin, cell nucleus, triolein, ceramide, melanin and water are the most important model components. We make available for download (see supplemental information) a database of Raman spectra for these eight components for others to use as a reference. Our model reveals the biochemical and structural makeup of normal, nonmelanoma and melanoma skin cancers, and precancers and paves the way for future development of this approach to noninvasive skin cancer diagnosis.
Keywords: (170.1610) Clinical applications; (170.1790) Confocal microscopy; (170.3880) Medical and biological imaging; (170.6510) Spectroscopy, tissue diagnostics; (300.6450) Spectroscopy, Raman.
Figures
References
-
- English D. R., Del Mar C., Burton R. C., “Factors influencing the number needed to excise: excision rates of pigmented lesions by general practitioners,” Med. J. Aust. 180(1), 16–19 (2004). - PubMed
-
- Barman I., Dingari N. C., Saha A., McGee S., Galindo L. H., Liu W., Plecha D., Klein N., Dasari R. R., Fitzmaurice M., “Application of Raman spectroscopy to identify microcalcifications and underlying breast lesions at stereotactic core needle biopsy,” Cancer Res. 73(11), 3206–3215 (2013).10.1158/0008-5472.CAN-12-2313 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
