Vitamin D3 ameliorates podocyte injury through the nephrin signalling pathway
- PMID: 28664547
- PMCID: PMC5618699
- DOI: 10.1111/jcmm.13180
Vitamin D3 ameliorates podocyte injury through the nephrin signalling pathway
Abstract
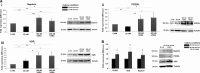
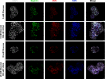
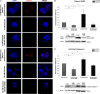
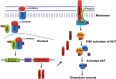
Renal podocytes form the main filtration barrier possessing unique phenotype maintained by proteins including podocalyxin and nephrin, which are modulated in pathological conditions. In diabetic nephropathy (DN), podocytes become structurally and functionally compromised. Nephrin, a structural backbone protein of the slit diaphragm, acts as regulator of podocyte intracellular signalling with renoprotective role. Vitamin D3 through its receptor, VDR, provides renal protection in DN but limited data exist about its effect on podocytes. In this study, we used isolated rat glomeruli to assess podocalyxin and nephrin expression after treatment with the 1,25-dihydroxyvitamin D3 analogue paricalcitol in the presence of normal and diabetic glucose levels. The role of 1,25-dihydroxyvitamin D3 (calcitriol) and its analogue, paricalcitol, on podocyte morphology and survival was also investigated in the streptozotocin (STZ)-diabetic animal model. In our ex vivo model, glomeruli exhibited high glucose-mediated down-regulation of podocalyxin, and nephrin, while paricalcitol reversed the high glucose-induced decrease of nephrin and podocalyxin expression. Paricalcitol treatment enhanced VDR expression and promoted VDR and RXR co-localization in the nucleus. Our data also indicated that hyperglycaemia impaired survival of cultured glomeruli and suggested that the implemented nephrin down-regulation was reversed by paricalcitol treatment, initiating Akt signal transduction which may be involved in glomerular survival. Our findings were further verified in vivo, as in the STZ-diabetic animal model, calcitriol and paricalcitol treatment resulted in significant amelioration of hyperglycaemia and restoration of nephrin signalling, suggesting that calcitriol and paricalcitol may provide molecular bases for protection against loss of the permselective renal barrier in DN.
Keywords: Diabetic nephropathy; STZ; Vitamin D3; Vitamin D3 Receptor; glomerulus; high glucose; nephrin; paricalcitol; podocalyxin.
© 2017 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures




Similar articles
-
Vitamin D receptor activators upregulate and rescue podocalyxin expression in high glucose-treated human podocytes.Nephron Exp Nephrol. 2012;122(1-2):36-50. doi: 10.1159/000346562. Epub 2013 Mar 14. Nephron Exp Nephrol. 2012. PMID: 23548800
-
Beyond proteinuria: VDR activation reduces renal inflammation in experimental diabetic nephropathy.Am J Physiol Renal Physiol. 2012 Mar 15;302(6):F647-57. doi: 10.1152/ajprenal.00090.2011. Epub 2011 Dec 14. Am J Physiol Renal Physiol. 2012. PMID: 22169009
-
The Therapeutic Effect of Active Vitamin D Supplementation in Preventing the Progression of Diabetic Nephropathy in a Diabetic Mouse Model.J Diabetes Res. 2020 Nov 26;2020:7907605. doi: 10.1155/2020/7907605. eCollection 2020. J Diabetes Res. 2020. PMID: 33294462 Free PMC article.
-
Urine markers of podocyte dysfunction: a review of podocalyxin and nephrin in selected glomerular diseases.Biomark Med. 2018 Aug;12(8):927-935. doi: 10.2217/bmm-2018-0152. Epub 2018 Jul 6. Biomark Med. 2018. PMID: 29976076 Review.
-
Role of nephrin in renal disease including diabetic nephropathy.Semin Nephrol. 2002 Sep;22(5):393-8. doi: 10.1053/snep.2002.34724. Semin Nephrol. 2002. PMID: 12224046 Review.
Cited by
-
Chronic constant light exposure aggravates high fat diet-induced renal injury in rats.Front Endocrinol (Lausanne). 2022 Jul 29;13:900392. doi: 10.3389/fendo.2022.900392. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35966094 Free PMC article.
-
The Role of Vitamin D in Diabetic Nephropathy: A Translational Approach.Int J Mol Sci. 2022 Jan 12;23(2):807. doi: 10.3390/ijms23020807. Int J Mol Sci. 2022. PMID: 35054991 Free PMC article. Review.
-
Calcitriol Treatment Attenuates Uric Acid-Induced Kidney Injury via Super Oxide Dismutase-1 (SOD-1) Upregulation and Fibrosis Reduction.Iran Biomed J. 2021 Nov 1;25(6):417-25. doi: 10.52547/ibj.25.6.417. Iran Biomed J. 2021. PMID: 34641645 Free PMC article.
-
The ShGlomAssay Combines High-Throughput Drug Screening With Downstream Analyses and Reveals the Protective Role of Vitamin D3 and Calcipotriol on Podocytes.Front Cell Dev Biol. 2022 May 16;10:838086. doi: 10.3389/fcell.2022.838086. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35652093 Free PMC article.
-
Association between Vitamin D Status and Mortality among Adults with Diabetic Kidney Disease.J Diabetes Res. 2022 May 9;2022:9632355. doi: 10.1155/2022/9632355. eCollection 2022. J Diabetes Res. 2022. PMID: 35586117 Free PMC article.
References
-
- Marco MP, Craver L, Betriu A, et al Influence of vitamin D receptor gene polymorphisms on mortality risk in hemodialysis patients. Am J Kidney Dis. 2001; 38: 965–74. - PubMed
-
- Tentori F, Hunt WC, Stidley CA, et al Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006; 70: 1858–65. - PubMed
-
- Wang AY. Cardiovascular risk in diabetic end‐stage renal disease patients. J Diabetes. 2011; 3: 119–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

