A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours
- PMID: 28664912
- PMCID: PMC5572165
- DOI: 10.1038/bjc.2017.199
A combined biomarker panel shows improved sensitivity for the early detection of ovarian cancer allowing the identification of the most aggressive type II tumours
Abstract
Background: There is an urgent need for biomarkers for the early detection of ovarian cancer (OC). The purpose of this study was to assess whether changes in serum levels of lecithin-cholesterol acyltransferase (LCAT), sex hormone-binding globulin (SHBG), glucose-regulated protein, 78 kDa (GRP78), calprotectin and insulin-like growth factor-binding protein 2 (IGFBP2) are observed before clinical presentation and to assess the performance of these markers alone and in combination with CA125 for early detection.
Methods: This nested case-control study used samples from the United Kingdom Collaborative Trial of Ovarian Cancer Screening trial. The sample set consisted of 482 serum samples from 49 OC subjects and 31 controls, with serial samples spanning up to 7 years pre-diagnosis. The set was divided into the following: (I) a discovery set, which included all women with only two samples from each woman, the first at<14 months and the second at >32 months to diagnosis; and (ii) a corroboration set, which included all the serial samples from the same women spanning the 7-year period. Lecithin-cholesterol acyltransferase, SHBG, GRP78, calprotectin and IGFBP2 were measured using ELISA. The performance of the markers to detect cancers pre-diagnosis was assessed.
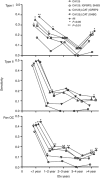
Results: A combined threshold model IGFBP2 >78.5 ng ml-1 : LCAT <8.831 μg ml-1 : CA125 >35 U ml-1 outperformed CA125 alone for the earlier detection of OC. The threshold model was able to identify the most aggressive Type II cancers. In addition, it increased the lead time by 5-6 months and identified 26% of Type I subjects and 13% of Type II subjects that were not identified by CA125 alone.
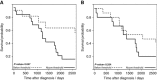
Conclusions: Combined biomarker panels (IGFBP2, LCAT and CA125) outperformed CA125 up to 3 years pre-diagnosis, identifying cancers missed by CA125, providing increased diagnostic lead times for Type I and Type II OC. The model identified more aggressive Type II cancers, with women crossing the threshold dying earlier, indicating that these markers can improve on the sensitivity of CA125 alone for the early detection of OC.
Conflict of interest statement
The authors declare the following potential conflict of interest. Both IJ and UM have a financial interest through UCL Business and Abcodia Ltd in the commercial use of UKCTOCS samples. IJ is a Non-Executive Director and Consultant to Abcodia Ltd and a Director of Women’s Health Specialists Ltd. All other authors declare no conflicts of interest.
Figures


Similar articles
-
The diagnostic performance of CA125 for the detection of ovarian and non-ovarian cancer in primary care: A population-based cohort study.PLoS Med. 2020 Oct 28;17(10):e1003295. doi: 10.1371/journal.pmed.1003295. eCollection 2020 Oct. PLoS Med. 2020. PMID: 33112854 Free PMC article.
-
Validation of a Novel Biomarker Panel for the Detection of Ovarian Cancer.Cancer Epidemiol Biomarkers Prev. 2016 Sep;25(9):1333-40. doi: 10.1158/1055-9965.EPI-15-1299. Epub 2016 Jul 22. Cancer Epidemiol Biomarkers Prev. 2016. PMID: 27448593 Free PMC article.
-
Comparison of candidate serologic markers for type I and type II ovarian cancer.Gynecol Oncol. 2011 Sep;122(3):560-6. doi: 10.1016/j.ygyno.2011.05.039. Epub 2011 Jun 24. Gynecol Oncol. 2011. PMID: 21704359 Free PMC article.
-
New tumor markers: CA125 and beyond.Int J Gynecol Cancer. 2005 Nov-Dec;15 Suppl 3:274-81. doi: 10.1111/j.1525-1438.2005.00441.x. Int J Gynecol Cancer. 2005. PMID: 16343244 Review.
-
Screening for ovarian cancer in the general population.Best Pract Res Clin Obstet Gynaecol. 2012 Apr;26(2):243-56. doi: 10.1016/j.bpobgyn.2011.11.006. Epub 2011 Dec 17. Best Pract Res Clin Obstet Gynaecol. 2012. PMID: 22182415 Review.
Cited by
-
Nanotechnology and Immunotherapy in Ovarian Cancer: Tracing New Landscapes.J Pharmacol Exp Ther. 2019 Sep;370(3):636-646. doi: 10.1124/jpet.118.254979. Epub 2019 Feb 8. J Pharmacol Exp Ther. 2019. PMID: 30737357 Free PMC article. Review.
-
Proteomics-driven discovery of LCAT as a novel biomarker for liver metastasis in colorectal cancer.BMC Cancer. 2025 Mar 15;25(1):480. doi: 10.1186/s12885-025-13882-x. BMC Cancer. 2025. PMID: 40089671 Free PMC article.
-
CD5L-associated gene analyses highlight the dysregulations, prognostic effects, immune associations, and drug-sensitivity predicative potentials of LCAT and CDC20 in hepatocellular carcinoma.Cancer Cell Int. 2022 Dec 9;22(1):393. doi: 10.1186/s12935-022-02820-7. Cancer Cell Int. 2022. PMID: 36494696 Free PMC article.
-
An Integrative Data Mining and Omics-Based Translational Model for the Identification and Validation of Oncogenic Biomarkers of Pancreatic Cancer.Cancers (Basel). 2019 Jan 29;11(2):155. doi: 10.3390/cancers11020155. Cancers (Basel). 2019. PMID: 30700038 Free PMC article.
-
High-throughput proteomics of breast cancer interstitial fluid: identification of tumor subtype-specific serologically relevant biomarkers.Mol Oncol. 2021 Feb;15(2):429-461. doi: 10.1002/1878-0261.12850. Epub 2021 Jan 4. Mol Oncol. 2021. PMID: 33176066 Free PMC article. Clinical Trial.
References
-
- Baron-Hay S, Boyle F, Ferrier A, Scott C (2004) Elevated serum insulin-like growth factor binding protein-2 as a prognostic marker in patients with ovarian cancer. Clin Cancer Res 10(5): 1796–1806. - PubMed
-
- Bristow RE, Smith A, Zhang Z, Chan DW, Crutcher G, Fung ET, Munroe DG (2013) Ovarian malignancy risk stratification of the adnexal mass using a multivariate index assay. Gynecol Oncol 128(2): 252–259. - PubMed
-
- Cramer DW, Bast RC, Berg CD, Diamandis EP, Godwin AK, Hartge P, Lokshin AE, Lu KH, McIntosh MW, Mor G, Patriotis C, Pinsky PF, Thornquist MD, Scholler N, Skates SJ, Sluss PM, Srivastava S, Ward DC, Zhang Z, Zhu CS, Urban N (2011) Ovarian cancer biomarker performance in prostate, lung, colorectal, and ovarian cancer screening trial specimens. Cancer Prev Res (Phila) 4(3): 365–374. - PMC - PubMed
-
- CRUK (2014) Ovarian Cancer Survival Statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/st....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

