Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors
- PMID: 28665051
- PMCID: PMC5581522
- DOI: 10.1111/cas.13307
Phase 1 dose-escalation study of single-agent veliparib in Japanese patients with advanced solid tumors
Abstract
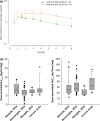
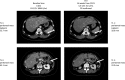
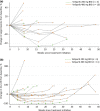
Veliparib (ABT-888) is a potent, orally bioavailable poly(ADP-ribose) polymerase-1 and -2 inhibitor. This phase 1 study evaluated the tolerability, pharmacokinetic profile, safety, and preliminary antitumor activity of single-agent veliparib in Japanese patients with advanced solid tumors. Eligible patients were assigned to treatment with veliparib at 200 or 400 mg dose; veliparib was self-administered orally twice daily on days 1-28 of 28-day cycles. Dose escalation, following a 3 + 3 design, defined dose-limiting toxicities, the maximum tolerated dose, and the recommended phase 2 dose. Sixteen patients were enrolled (median age, 59 years). Fourteen patients had high-grade serous ovarian cancer, one had primary peritoneal cancer, and one had BRCA-mutated breast cancer. The most frequent treatment-emergent adverse events were nausea and vomiting (93.8% each), decreased appetite (62.5%), abdominal pain, diarrhea, and malaise (31.3% each). A grade ≥3 toxicity was observed in 50% of patients; one patient each in the 200 mg (n = 4) and 400 mg (n = 12) cohorts experienced serious adverse events. Dose-limiting toxicities were observed for one patient at the 400 mg dose. No toxicities leading to death were reported. The recommended phase 2 dose was defined as 400 mg twice daily. The veliparib pharmacokinetic profile was consistent with that reported for the Western population. Two patients, both with ovarian cancer, had a RECIST partial response. Veliparib monotherapy showed manageable tolerability and safety profiles and a predictable pharmacokinetic profile at a 400 mg twice-daily dose, and supports the inclusion of Japanese patients in the multinational phase 3 study (NCT02470585).
Keywords: High-grade serous ovarian cancer; Japanese; phase 1; poly(ADP-ribose) polymerase; veliparib.
© 2017 The Authors. Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Figures
References
-
- National Cancer Institute . Surveillance, epidemiology, and end results program. Cancer stat facts: Ovarian cancer. [Cited 29 March 2017.] Available from URL: https://seer.cancer.gov/statfacts/html/ovary.html.
-
- International Agency for Research on Cancer . World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide in 2012. [Cited 29 March 2017.] Available from URL: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
-
- Foundation for Promotion of Cancer Research . Cancer statistics in Japan – 2015. 2015. [Cited 29 March 2017.] Available from URL: http://ganjoho.jp/data/reg_stat/statistics/brochure/2015/cancer_statisti....
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

