Biological Fate of Fe₃O₄ Core-Shell Mesoporous Silica Nanoparticles Depending on Particle Surface Chemistry
- PMID: 28665317
- PMCID: PMC5535228
- DOI: 10.3390/nano7070162
Biological Fate of Fe₃O₄ Core-Shell Mesoporous Silica Nanoparticles Depending on Particle Surface Chemistry
Abstract
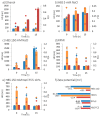
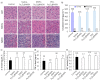
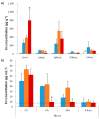
The biological fate of nanoparticles (NPs) for biomedical applications is highly dependent of their size and charge, their aggregation state and their surface chemistry. The chemical composition of the NPs surface influences their stability in biological fluids, their interaction with proteins, and their attraction to the cell membranes. In this work, core-shell magnetic mesoporous silica nanoparticles (Fe₃O₄@MSN), that are considered as potential theranostic candidates, are coated with polyethylene glycol (PEG) or 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC) lipid bilayer. Their biological fate is studied in comparison to the native NPs. The physicochemical properties of these three types of NPs and their suspension behavior in different media are investigated. The attraction to a membrane model is also evaluated using a supported lipid bilayer. The surface composition of NPs strongly influences their dispersion in biological fluids mimics, protein binding and their interaction with cell membrane. While none of these types of NPs is found to be toxic on mice four days after intravenous injection of a dose of 40 mg kg-1 of NPs, their surface coating nature influences the in vivo biodistribution. Importantly, NP coated with DMPC exhibit a strong accumulation in liver and a very low accumulation in lung in comparison with nude or PEG ones.
Keywords: biodistribution; cell-membrane interactions; nanoparticles; safety; surface coating.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

