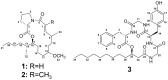
Tricholides A and B and Unnarmicin D: New Hybrid PKS-NRPS Macrocycles Isolated from an Environmental Collection of Trichodesmium thiebautii
- PMID: 28665343
- PMCID: PMC5532648
- DOI: 10.3390/md15070206
Tricholides A and B and Unnarmicin D: New Hybrid PKS-NRPS Macrocycles Isolated from an Environmental Collection of Trichodesmium thiebautii
Abstract
Bioassay-guided isolation of the lipophilic extract of Trichodesmium thiebautii bloom material led to the purification and structure characterization of two new hybrid polyketide-non-ribosomal peptide (PKS-NRPS) macrocyclic compounds, tricholides A and B (1 and 2). A third macrocyclic compound, unnarmicin D (3), was identified as a new depsipeptide in the unnarmicin family, given its structural similarity to the existing compounds in this group. The planar structures of 1-3 were determined using 1D and 2D NMR spectra and complementary spectroscopic and spectrometric procedures. The absolute configurations of the amino acid components of 1-3 were determined via acid hydrolysis, derivitization with Marfey's reagent and HPLC-UV comparison to authentic amino acid standards. The absolute configuration of the 3-hydroxydodecanoic acid moiety in 3 was determined using a modified Mosher's esterification procedure on a linear derivative of tricharmicin (4) and additionally by a comparison of 13C NMR shifts of 3 to known depsipeptides with β-hydroxy acid subunits. Tricholide B (2) showed moderate cytotoxicity to Neuro-2A murine neuroblastoma cells (EC50: 14.5 ± 6.2 μM).
Keywords: Trichodesmium thiebautii; cyanobacteria; depsipeptide; macrocycle.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Trichophycin A, a Cytotoxic Linear Polyketide Isolated from a Trichodesmium thiebautii Bloom.Mar Drugs. 2017 Jan 6;15(1):10. doi: 10.3390/md15010010. Mar Drugs. 2017. PMID: 28067831 Free PMC article.
-
Isolation of Isotrichophycin C and Trichophycins G-I from a Collection of Trichodesmium thiebautii.J Nat Prod. 2020 Sep 25;83(9):2664-2671. doi: 10.1021/acs.jnatprod.0c00550. Epub 2020 Aug 20. J Nat Prod. 2020. PMID: 32816476 Free PMC article.
-
Trichophycins B-F, Chlorovinylidene-Containing Polyketides Isolated from a Cyanobacterial Bloom.J Org Chem. 2018 Nov 2;83(21):13256-13266. doi: 10.1021/acs.joc.8b02070. Epub 2018 Oct 17. J Org Chem. 2018. PMID: 30280904 Free PMC article.
-
Structure revision of trichotoxin, a chlorinated polyketide isolated from a Trichodesmium thiebautii bloom.Tetrahedron Lett. 2016 Dec 28;57(52):5864-5867. doi: 10.1016/j.tetlet.2016.11.062. Epub 2016 Nov 15. Tetrahedron Lett. 2016. PMID: 32153305 Free PMC article.
-
Molecular Networking-Based Screening Led to the Discovery of a Cyclic Heptadepsipeptide from an Endolichenic Xylaria sp.J Nat Prod. 2022 Apr 22;85(4):972-979. doi: 10.1021/acs.jnatprod.1c01108. Epub 2022 Apr 6. J Nat Prod. 2022. PMID: 35385664 Review.
Cited by
-
Trichothiazole A, a dichlorinated polyketide containing an embedded thiazole isolated from Trichodesmium blooms.Tetrahedron Lett. 2017 Oct 25;58(43):4066-4068. doi: 10.1016/j.tetlet.2017.09.027. Epub 2017 Sep 17. Tetrahedron Lett. 2017. PMID: 32189813 Free PMC article.
-
A multi-omics reciprocal analysis for characterization of bacterial metabolism.Front Mol Biosci. 2025 Mar 20;12:1515276. doi: 10.3389/fmolb.2025.1515276. eCollection 2025. Front Mol Biosci. 2025. PMID: 40182618 Free PMC article.
-
Total Synthesis of Sanctolide A and Formal Synthesis of (2S)-Sanctolide A.J Org Chem. 2023 Jan 20;88(2):805-817. doi: 10.1021/acs.joc.2c01922. Epub 2023 Jan 5. J Org Chem. 2023. PMID: 36602547 Free PMC article.
-
Spatial and Temporal Resolution of Cyanobacterial Bloom Chemistry Reveals an Open-Ocean Trichodesmium thiebautii as a Talented Producer of Specialized Metabolites.Environ Sci Technol. 2024 Jun 4;58(22):9525-9535. doi: 10.1021/acs.est.3c10739. Epub 2024 May 17. Environ Sci Technol. 2024. PMID: 38758591 Free PMC article.
-
Impact of dehydration on the physiochemical properties of Nostoc calcicola BOT1 and its untargeted metabolic profiling through UHPLC-HRMS.Front Plant Sci. 2023 Jun 23;14:1147390. doi: 10.3389/fpls.2023.1147390. eCollection 2023. Front Plant Sci. 2023. PMID: 37426961 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous