A Novel Synthesis of the Efficient Anti-Coccidial Drug Halofuginone Hydrobromide
- PMID: 28665346
- PMCID: PMC6152095
- DOI: 10.3390/molecules22071086
A Novel Synthesis of the Efficient Anti-Coccidial Drug Halofuginone Hydrobromide
Abstract
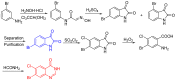
Background: Halofuginone hydrobromide (1) is recognized as an effective drug against several species of Eimeria (E.) in poultry. In this paper, we describe a convenient and low cost preparation method for the compound, as well as primary validation of its activity. Methods: First, 7-bromo-6-chloroquinazolin-4(3H)-one (2) was prepared from m-chlorotoluene by a conventional process, and then chloroacetone was creatively introduced in two steps. Finally, halofuginone hydrobromide (1) was obtained from 7-bromo-6-chloro-3-(3-cholroacetonyl) quinazolin-4(3H)-one (4) by a four-step reaction sequence including condensation, cyclization, deprotection and isomerization. The structures of the relative intermediates and target compound were characterized by melting point, IR, MS and ¹H-NMR. Besides, the protective effect of compound 1-supplemented chicken diet at doses of 6, 3 and 1.5 mg per 1 kg were evaluated on chickens infected with E. tenella, by reduction in mortality, weight loss, fecal oocyst excretion and gut pathology, respectively. Results: Halofuginone hydrobromide (1) was prepared successfully by and improved and innovative method based on traditional research. Moreover, the synthesized halofuginone hydrobromide significantly exhibited an anti-coccidial property. Conclusions: The fruitful work described in this Communication has resulted in halofuginone hydrobromide, which has a good pharmaceutical development prospects, becoming more available for large-scale production.
Keywords: 4(3H)-quinazolinone; asymmetric synthesis; febrifugine; halofuginone hydrobromide; piperidine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

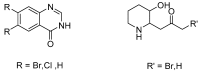



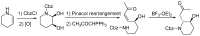


Similar articles
-
Anticoccidial effect of halofuginone hydrobromide against Eimeria tenella with associated histology.Parasitol Res. 2012 Aug;111(2):695-701. doi: 10.1007/s00436-012-2889-7. Epub 2012 Mar 14. Parasitol Res. 2012. PMID: 22415441 Clinical Trial.
-
Chemical synthesis of febrifugine and analogues.Bioorg Med Chem. 2018 May 15;26(9):2199-2220. doi: 10.1016/j.bmc.2018.04.027. Epub 2018 Apr 12. Bioorg Med Chem. 2018. PMID: 29681487 Review.
-
The chemistry and biology of febrifugine and halofuginone.Bioorg Med Chem. 2014 Apr 1;22(7):1993-2004. doi: 10.1016/j.bmc.2014.02.040. Epub 2014 Mar 1. Bioorg Med Chem. 2014. PMID: 24650700 Review.
-
Effect of Bidens pilosa on infection and drug resistance of Eimeria in chickens.Res Vet Sci. 2015 Feb;98:74-81. doi: 10.1016/j.rvsc.2014.11.002. Epub 2014 Nov 14. Res Vet Sci. 2015. PMID: 25440995 Clinical Trial.
-
Synthesis and comparison of antimalarial activity of febrifugine derivatives including halofuginone.Med Chem. 2009 May;5(3):293-300. doi: 10.2174/157340609788185846. Med Chem. 2009. PMID: 19442220
Cited by
-
Traditional medicine meets modern science: Halofuginone's role in combating autoimmune diseases.J Nat Med. 2025 Sep;79(5):1017-1029. doi: 10.1007/s11418-025-01927-1. Epub 2025 Jun 30. J Nat Med. 2025. PMID: 40586966 Review.
-
Molecular pathogenesis of Cryptosporidium and advancements in therapeutic interventions.Parasite. 2025;32:7. doi: 10.1051/parasite/2025001. Epub 2025 Feb 4. Parasite. 2025. PMID: 39902829 Free PMC article. Review.
-
Catalyst- and Solvent-Free Coupling of 2-Methyl Quinazolinones and Isatins: An Environmentally Benign Access of Diastereoselective Schizocommunin Analogues.ACS Omega. 2019 Jul 15;4(7):12146-12155. doi: 10.1021/acsomega.9b01514. eCollection 2019 Jul 31. ACS Omega. 2019. PMID: 31460329 Free PMC article.
References
-
- Emanuel W., Gerald B., Sidney K. Method for Treating Coccidiosis with Quinazolinones. US3,320,124. U.S. Patent. 1967 May 16;
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

