Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up
- PMID: 28665938
- PMCID: PMC5493332
- DOI: 10.1371/journal.pone.0179870
Trends and outcomes of late initiation of combination antiretroviral therapy driven by late presentation among HIV-positive Taiwanese patients in the era of treatment scale-up
Abstract
Objectives: The international and national HIV treatment guidelines in 2016 have focused on scaling up access to combination antiretroviral therapy (cART). We aimed to assess the trends and treatment outcomes of late cART initiation in Taiwan.
Methods: Between June 2012 and May 2016, we retrospectively included antiretroviral-naive HIV-positive adults who initiated cART. Late initiation was defined as when cART was initiated in patients with a CD4 count <200 cells/mm3 or having experienced AIDS-defining illnesses. The treatment outcomes were assessed up to 6 months after starting cART.
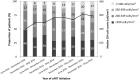
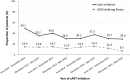
Results: We included 3655 HIV-positive patients, and the majority of the patients were male (95.4%) with a median age of 31 years and initiated non-nucleoside reverse-transcriptase inhibitor-containing regimens (87.0%). The median CD4 count at cART initiation increased from 207 cells/mm3 in 2012 to 298 cells/mm3 in 2016, and the overall proportion of late cART initiation decreased from 49.1% in 2012 to 29.0% in 2016 (P for trend <0.001). Late cART initiation mainly resulted from late presentation for HIV care and was associated with older age (per 1-year increase, adjusted odds ratio [AOR], 1.05; 95% CI, 1.04-1.06), HBsAg seropositivity (AOR, 1.31; 95% CI, 1.04-1.64), HIV care in central and southern Taiwan, initiating cART in earlier year, non-intravenous drug users (AOR, 1.96; 95% CI, 1.33-2.86), and negative hepatitis C serostatus (AOR, 1.47; 95% CI, 1.04-2.08). Compared with non-late initiators, late initiators had a higher rate of all-cause mortality (1.7% vs. 0.3%) and regimen modification due to virological failure (7.1% vs. 2.6%). The predicting factors of all-cause mortality were late cART initiation (adjusted hazard ratio [AHR], 5.40; 95% CI, 2.14-13.65) and older age (AHR, 1.06; 95% CI, 1.03-1.10).
Conclusions: While the proportion of late cART initiation decreased over time in Taiwan, late initiation remained in a substantial proportion of HIV-positive patients. The late initiators had higher risk for poor outcomes. The need for strategies to earlier detection of HIV infection and expediting cART initiation should be highlighted, especially among the older population.
Conflict of interest statement
Figures

References
-
- UNAIDS/WHO. Report on the global AIDS epidemic. 2013. Available from: http://www.unaids.org/en/resources/documents/2013/20130923_UNAIDS_Global.... Cited 10 May 2017.
-
- Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2016. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. Cited 10 May 2017.
-
- Danel C, Moh R, Gabillard D, Badje A, Le Carrou J, Ouassa T, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. The New England journal of medicine. 2015;373(9):808–22. doi: 10.1056/NEJMoa1507198 - DOI - PubMed
-
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Antiretroviral therapy for the prevention of HIV-1 transmission. The New England journal of medicine. 2016;375(9):830–9. doi: 10.1056/NEJMoa1600693 - DOI - PMC - PubMed
-
- UNAIDS/WHO. 90-90-90: An ambitious treatment target to help end the AIDS epidemic. 2014. Available from: http://www.unaids.org/en/resources/documents/2014/90-90-90. Cited 10 May 2017.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

