Increased migration of antigen presenting cells to newly-formed lymphatic vessels in transplanted kidneys by glycol-split heparin
- PMID: 28665959
- PMCID: PMC5493359
- DOI: 10.1371/journal.pone.0180206
Increased migration of antigen presenting cells to newly-formed lymphatic vessels in transplanted kidneys by glycol-split heparin
Abstract
Background: Chronic renal transplant dysfunction is characterized by loss of renal function and tissue remodeling, including chronic inflammation and lymph vessel formation. Proteoglycans are known for their chemokine presenting capacity. We hypothesize that interruption of the lymphatic chemokine-proteoglycan interaction interferes with the lymphatic outflow of leukocytes from the renal graft and might decrease the anti-graft allo-immune response.
Methods: In a rat renal chronic transplant dysfunction model (female Dark-Agouti to male Wistar Furth), chemokines were profiled by qRT-PCR in microdissected tubulo-interstitial tissue. Disruption of lymphatic chemokine-proteoglycan interaction was studied by (non-anticoagulant) heparin-derived polysaccharides in vitro and in renal allografts. The renal allograft function was assessed by rise in plasma creatinine and urea.
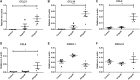
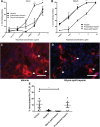
Results: Within newly-formed lymph vessels of transplanted kidneys, numerous CD45+ leukocytes were found, mainly MHCII+, ED-1-, IDO-, HIS14-, CD103- antigen presenting cells, most likely representing a subset of dendritic cells. Treatment of transplanted rats with regular heparin and two different (non-)anticoagulant heparin derivatives revealed worsening of kidney function only in the glycol-split heparin treated group despite a two-fold reduction of tubulo-interstitial leukocytes (p<0.02). Quantitative digital image analysis however revealed increased numbers of intra-lymphatic antigen-presenting cells only in the glycol-split heparin group (p<0.01). The number of intra-lymphatic leukocytes significantly correlates with plasma creatinine and urea, and inversely with creatinine clearance.
Conclusions: Treatment of transplanted rats with glycol-split heparin significantly increases the number of intra-lymphatic antigen presenting cells, by increased renal diffusion of lymphatic chemokines, thereby increasing the activation and recruitment of antigen presenting cells towards the lymph vessel. This effect is unwanted in the transplantation setting, but might be advantageous in e.g., dendritic cell vaccination.
Conflict of interest statement
Figures





Similar articles
-
Differential expression of proteoglycans in tissue remodeling and lymphangiogenesis after experimental renal transplantation in rats.PLoS One. 2010 Feb 5;5(2):e9095. doi: 10.1371/journal.pone.0009095. PLoS One. 2010. PMID: 20140097 Free PMC article.
-
Targeting lymphatic vessel activation and CCL21 production by vascular endothelial growth factor receptor-3 inhibition has novel immunomodulatory and antiarteriosclerotic effects in cardiac allografts.Circulation. 2010 Mar 30;121(12):1413-22. doi: 10.1161/CIRCULATIONAHA.109.910703. Epub 2010 Mar 15. Circulation. 2010. PMID: 20231530
-
Clinicopathological study of expression of lymphatic vessels in renal allograft biopsy after treatment for acute rejection.Transplant Proc. 2009 Dec;41(10):4154-8. doi: 10.1016/j.transproceed.2009.09.067. Transplant Proc. 2009. PMID: 20005358
-
Dendritic cell migration through the lymphatic vasculature to lymph nodes.Adv Immunol. 2013;120:51-68. doi: 10.1016/B978-0-12-417028-5.00002-8. Adv Immunol. 2013. PMID: 24070380 Review.
-
Dendritic-cell trafficking to lymph nodes through lymphatic vessels.Nat Rev Immunol. 2005 Aug;5(8):617-28. doi: 10.1038/nri1670. Nat Rev Immunol. 2005. PMID: 16056255 Review.
Cited by
-
Lymphatic Reconstruction in Kidney Allograft Aggravates Chronic Rejection by Promoting Alloantigen Presentation.Front Immunol. 2021 Dec 9;12:796260. doi: 10.3389/fimmu.2021.796260. eCollection 2021. Front Immunol. 2021. PMID: 34956231 Free PMC article.
-
Beyond a Passive Conduit: Implications of Lymphatic Biology for Kidney Diseases.J Am Soc Nephrol. 2020 Jun;31(6):1178-1190. doi: 10.1681/ASN.2019121320. Epub 2020 Apr 15. J Am Soc Nephrol. 2020. PMID: 32295825 Free PMC article. Review.
-
Prevention of Triglyceridemia by (Non-)Anticoagulant Heparin(oids) Does Not Preclude Transplant Vasculopathy and Glomerulosclerosis.Front Cell Dev Biol. 2022 Mar 7;10:798088. doi: 10.3389/fcell.2022.798088. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35345850 Free PMC article.
-
The lymphatics in kidney health and disease.Nat Rev Nephrol. 2021 Oct;17(10):655-675. doi: 10.1038/s41581-021-00438-y. Epub 2021 Jun 22. Nat Rev Nephrol. 2021. PMID: 34158633 Review.
-
Analyzing Lymphatic Vessel Patterning in Adult Tissue.Methods Mol Biol. 2022;2441:85-94. doi: 10.1007/978-1-0716-2059-5_7. Methods Mol Biol. 2022. PMID: 35099730
References
-
- Chapman JR, O'Connell PJ, Nankivell BJ. Chronic renal allograft dysfunction. J Am Soc Nephrol. 2005;16: 3015–3026. doi: 10.1681/ASN.2005050463 - DOI - PubMed
-
- Solez K. Making global transplantation pathology standards truly global. Am J Transplant. 2007;7: 2834 doi: 10.1111/j.1600-6143.2007.02001.x - DOI - PubMed
-
- Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8: 753–760. doi: 10.1111/j.1600-6143.2008.02159.x - DOI - PubMed
-
- Kerjaschki D. The crucial role of macrophages in lymphangiogenesis. J Clin Invest. 2005;115: 2316–2319. doi: 10.1172/JCI26354 - DOI - PMC - PubMed
-
- Yazdani S, Navis G, Hillebrands JL, van Goor H, van den Born J. Lymphangiogenesis in renal diseases: passive bystander or active participant? Expert Rev Mol Med. 2014;16: e15 doi: 10.1017/erm.2014.18 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

