Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial
- PMID: 28665989
- PMCID: PMC5493377
- DOI: 10.1371/journal.pone.0180328
Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: A pilot randomised controlled trial
Abstract
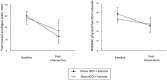
A randomised, assessor- and participant-blind, sham-controlled trial was conducted to assess the safety and feasibility of adding transcranial direct current stimulation (tDCS) to quadriceps strengthening exercise in knee osteoarthritis (OA), and provide data to inform a fully powered trial. Participants were randomised to receive active tDCS+exercise (AT+EX) or sham tDCS+exercise (ST+EX) twice weekly for 8 weeks whilst completing home exercises twice per week. Feasibility, safety, patient-perceived response, pain, function, pressure pain thresholds (PPTs) and conditioned pain modulation (CPM) were assessed before and after treatment. Fifty-seven people were screened for eligibility. Thirty (52%) entered randomisation and 25 (84%) completed the trial. One episode of headache in the AT+EX group was reported. Pain reduced in both groups following treatment (AT+EX: p<0.001, partial η2 = 0.55; ST+EX: p = 0.026, partial η2 = 0.18) but no between-group differences were observed (p = 0.18, partial η2 = 0.08). Function improved in the AT+EX (p = 0.01, partial η2 = 0.22), but not the ST+EX (p = 0.16, partial η2 = 0.08) group, between-group differences did not reach significance (p = 0.28, partial η2 = 0.052). AT+EX produced greater improvements in PPTs than ST+EX (p<0.05) (superolateral knee: partial η2 = 0.17; superior knee: partial η2 = 0.3; superomedial knee: partial η2 = 0.26). CPM only improved in the AT+EX group but no between-group difference was observed (p = 0.054, partial η2 = 0.158). This study provides the first feasibility and safety data for the addition of tDCS to quadriceps strengthening exercise in knee OA. Our data suggest AT+EX may improve pain, function and pain mechanisms beyond that of ST+EX, and provides support for progression to a fully powered randomised controlled trial.
Conflict of interest statement
Figures



References
-
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. Epub 2012/12/19. doi: 10.1016/S0140-6736(12)61729-2 . - DOI - PMC - PubMed
-
- Gierisch JM, Myers ER, Schmit KM, McCrory DC, Coeytaux RR, Crowley MJ, et al. Prioritization of patient-centered comparative effectiveness research for osteoarthritis. Annals of internal medicine. 2014;160(12):836–41. doi: 10.7326/M14-0318 . - DOI - PubMed
-
- McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363–88. Epub 2014/01/28. doi: 10.1016/j.joca.2014.01.003 . - DOI - PubMed
-
- Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465–74. Epub 2012/05/09. . - PubMed
-
- Fransen M, McConnell S, Harmer AR, Van der Esch M, Simic M, Bennell KL. Exercise for osteoarthritis of the knee. The Cochrane database of systematic reviews. 2015;1:Cd004376 Epub 2015/01/09. doi: 10.1002/14651858.CD004376.pub3 . - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

