Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension
- PMID: 28666030
- PMCID: PMC5493402
- DOI: 10.1371/journal.pone.0180455
Contribution of reactive oxygen species to the pathogenesis of pulmonary arterial hypertension
Abstract
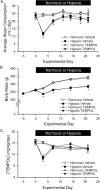
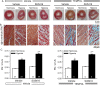
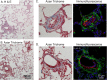
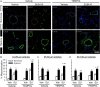
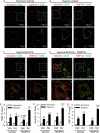
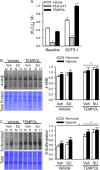
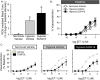
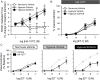
Pulmonary arterial hypertension is associated with a decreased antioxidant capacity. However, neither the contribution of reactive oxygen species to pulmonary vasoconstrictor sensitivity, nor the therapeutic efficacy of antioxidant strategies in this setting are known. We hypothesized that reactive oxygen species play a central role in mediating both vasoconstrictor and arterial remodeling components of severe pulmonary arterial hypertension. We examined the effect of the chemical antioxidant, TEMPOL, on right ventricular systolic pressure, vascular remodeling, and enhanced vasoconstrictor reactivity in both chronic hypoxia and hypoxia/SU5416 rat models of pulmonary hypertension. SU5416 is a vascular endothelial growth factor receptor antagonist and the combination of chronic hypoxia/SU5416 produces a model of severe pulmonary arterial hypertension with vascular plexiform lesions/fibrosis that is not present with chronic hypoxia alone. The major findings from this study are: 1) compared to hypoxia alone, hypoxia/SU5416 exposure caused more severe pulmonary hypertension, right ventricular hypertrophy, adventitial lesion formation, and greater vasoconstrictor sensitivity through a superoxide and Rho kinase-dependent Ca2+ sensitization mechanism. 2) Chronic hypoxia increased medial muscularization and superoxide levels, however there was no effect of SU5416 to augment these responses. 3) Treatment with TEMPOL decreased right ventricular systolic pressure in both hypoxia and hypoxia/SU5416 groups. 4) This effect of TEMPOL was associated with normalization of vasoconstrictor responses, but not arterial remodeling. Rather, medial hypertrophy and adventitial fibrotic lesion formation were more pronounced following chronic TEMPOL treatment in hypoxia/SU5416 rats. Our findings support a major role for reactive oxygen species in mediating enhanced vasoconstrictor reactivity and pulmonary hypertension in both chronic hypoxia and hypoxia/SU5416 rat models, despite a paradoxical effect of antioxidant therapy to exacerbate arterial remodeling in animals with severe pulmonary arterial hypertension in the hypoxia/SU5416 model.
Conflict of interest statement
Figures



References
-
- Howell K, Preston RJ, McLoughlin P. Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. The Journal of Physiology. 2003;547(1):133–45. doi: 10.1113/jphysiol.2002.030676 - DOI - PMC - PubMed
-
- Taraseviciene-Stewart L, Kasahara Y, Alger L, Hirth P, Mc Mahon G, Waltenberger J, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. The FASEB Journal. 2001;15(2):427–38. doi: 10.1096/fj.00-0343com - DOI - PubMed
-
- Aggarwal S, Gross CM, Sharma S, Fineman JR, Black SM. Reactive oxygen species in pulmonary vascular remodeling. Comprehensive Physiology. 2013;3(3):1011–34. doi: 10.1002/cphy.c120024 - DOI - PMC - PubMed
-
- Voelkel NF, Bogaard HJ, Husseini AA, Farkas L, Gomez-Arroyo J, Natarajan R. Antioxidants for the Treatment of Patients with Severe Angioproliferative Pulmonary Hypertension? Antioxidants & Redox Signaling. 2012;18(14):1810–7. doi: 10.1089/ars.2012.4828 - DOI - PubMed
-
- Wong C-M, Bansal G, Pavlickova L, Marcocci L, Suzuki YJ. Reactive Oxygen Species and Antioxidants in Pulmonary Hypertension. Antioxidants & Redox Signaling. 2013;18(14):1789–96. doi: 10.1089/ars.2012.4568 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

