Bacterial Cell Mechanics
- PMID: 28666084
- PMCID: PMC6260806
- DOI: 10.1021/acs.biochem.7b00346
Bacterial Cell Mechanics
Abstract
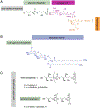
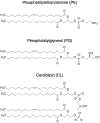
Cellular mechanical properties play an integral role in bacterial survival and adaptation. Historically, the bacterial cell wall and, in particular, the layer of polymeric material called the peptidoglycan were the elements to which cell mechanics could be primarily attributed. Disrupting the biochemical machinery that assembles the peptidoglycan (e.g., using the β-lactam family of antibiotics) alters the structure of this material, leads to mechanical defects, and results in cell lysis. Decades after the discovery of peptidoglycan-synthesizing enzymes, the mechanisms that underlie their positioning and regulation are still not entirely understood. In addition, recent evidence suggests a diverse group of other biochemical elements influence bacterial cell mechanics, may be regulated by new cellular mechanisms, and may be triggered in different environmental contexts to enable cell adaptation and survival. This review summarizes the contributions that different biomolecular components of the cell wall (e.g., lipopolysaccharides, wall and lipoteichoic acids, lipid bilayers, peptidoglycan, and proteins) make to Gram-negative and Gram-positive bacterial cell mechanics. We discuss the contribution of individual proteins and macromolecular complexes in cell mechanics and the tools that make it possible to quantitatively decipher the biochemical machinery that contributes to bacterial cell mechanics. Advances in this area may provide insight into new biology and influence the development of antibacterial chemotherapies.
Figures




References
-
- Louise Meyer R, Zhou X, Tang L, Arpanaei A, Kingshott P, and Besenbacher F (2010) Immobilisation of living bacteria for AFM imaging under physiological conditions. Ultramicroscopy 110, 1349–1357. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

