IFNγ-Dependent Tissue-Immune Homeostasis Is Co-opted in the Tumor Microenvironment
- PMID: 28666115
- PMCID: PMC5569303
- DOI: 10.1016/j.cell.2017.06.016
IFNγ-Dependent Tissue-Immune Homeostasis Is Co-opted in the Tumor Microenvironment
Abstract
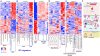
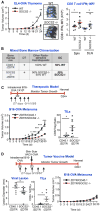
Homeostatic programs balance immune protection and self-tolerance. Such mechanisms likely impact autoimmunity and tumor formation, respectively. How homeostasis is maintained and impacts tumor surveillance is unknown. Here, we find that different immune mononuclear phagocytes share a conserved steady-state program during differentiation and entry into healthy tissue. IFNγ is necessary and sufficient to induce this program, revealing a key instructive role. Remarkably, homeostatic and IFNγ-dependent programs enrich across primary human tumors, including melanoma, and stratify survival. Single-cell RNA sequencing (RNA-seq) reveals enrichment of homeostatic modules in monocytes and DCs from human metastatic melanoma. Suppressor-of-cytokine-2 (SOCS2) protein, a conserved program transcript, is expressed by mononuclear phagocytes infiltrating primary melanoma and is induced by IFNγ. SOCS2 limits adaptive anti-tumoral immunity and DC-based priming of T cells in vivo, indicating a critical regulatory role. These findings link immune homeostasis to key determinants of anti-tumoral immunity and escape, revealing co-opting of tissue-specific immune development in the tumor microenvironment.
Keywords: IFNγ; dendritic cells; differentiation; homeostasis; immunotherapy; melanoma; suppressor-of-cytokine-signaling 2 (SOCS2); tissue mononuclear phagocytes; tolerance; tumor microenvironment.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures





Comment in
-
IFN-γ in tissue-immune homeostasis and antitumor immunity.Cell Mol Immunol. 2018 May;15(5):531-532. doi: 10.1038/cmi.2017.95. Epub 2017 Oct 2. Cell Mol Immunol. 2018. PMID: 28967878 Free PMC article. No abstract available.
References
-
- Anandasabapathy N, Victora GD, Meredith M, Feder R, Dong B, Kluger C, Yao K, Dustin ML, Nussenzweig MC, Steinman RM, Liu K. Flt3L controls the development of radiosensitive dendritic cells in the meninges and choroid plexus of the steady-state mouse brain. J Exp Med. 2011;208:1695–1705. - PMC - PubMed
-
- Baratin M, Foray C, Demaria O, Habbeddine M, Pollet E, Maurizio J, Verthuy C, Davanture S, Azukizawa H, Flores-Langarica A, et al. Homeostatic NF-κB Signaling in Steady-State Migratory Dendritic Cells Regulates Immune Homeostasis and Tolerance. Immunity. 2015;42:627–639. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

