Neoadjuvant radiotherapy of early-stage breast cancer and long-term disease-free survival
- PMID: 28666457
- PMCID: PMC5493088
- DOI: 10.1186/s13058-017-0870-1
Neoadjuvant radiotherapy of early-stage breast cancer and long-term disease-free survival
Abstract
Background: Compared with surgery alone, postoperative adjuvant radiotherapy (RT) improves relapse-free survival of patients with early-stage breast cancer. We evaluated the long-term overall and disease-free survival rates of neoadjuvant (presurgical) versus adjuvant RT in early-stage breast cancer patients.
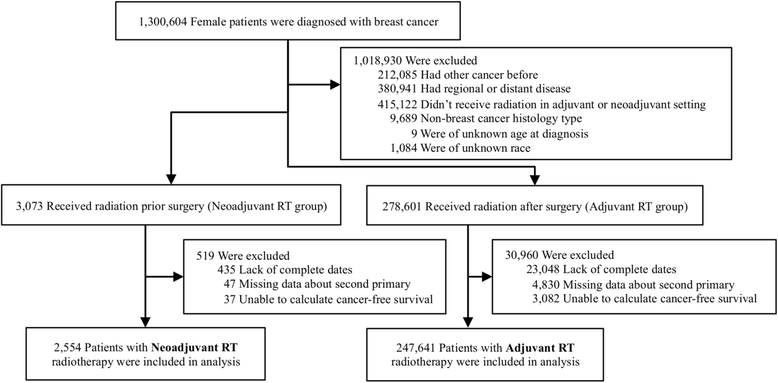
Methods: We used the Surveillance, Epidemiology, and End Results (SEER) database provided by the National Institutes of Health to derive an analytic dataset of 250,195 female patients with early-stage breast cancer who received RT before (n = 2554; 1.02%) or after (n = 247,641; 98.98%) surgery. Disease-free survival, defined as time to diagnosis of a second primary tumor at any location, was calculated from automated patient identification matching of all SEER records.
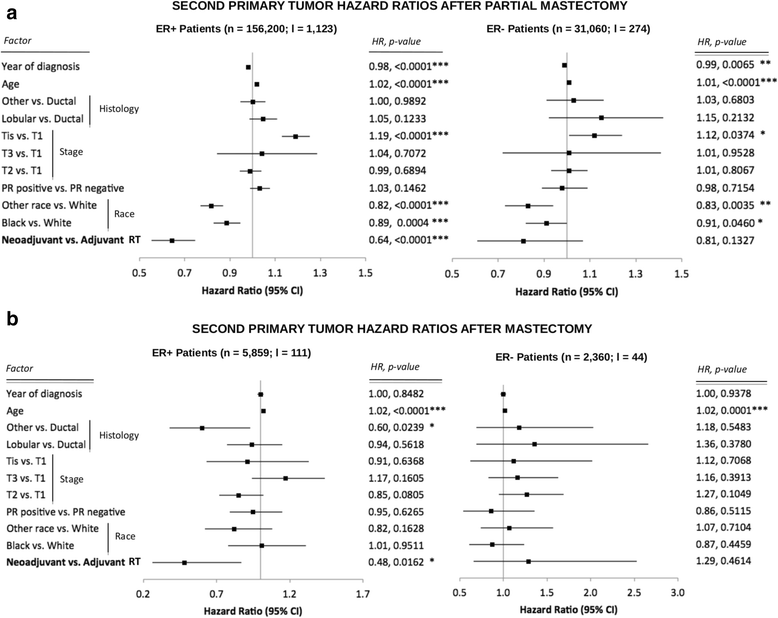
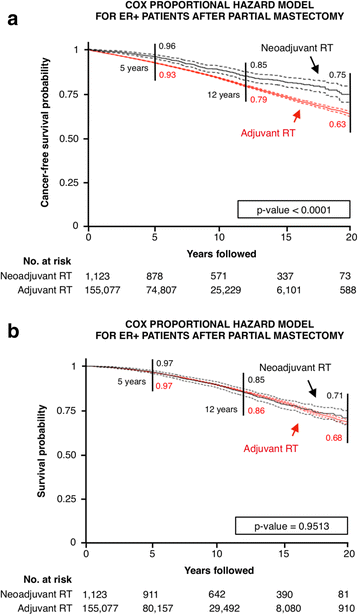
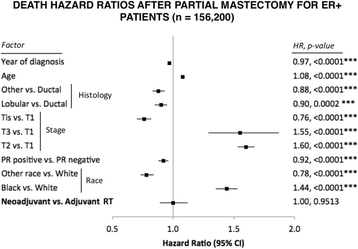
Results: Partial and complete mastectomies were performed in 94.4% and 5.6% of patients, respectively. In the largest cohort of estrogen receptor-positive women who underwent partial mastectomy, the HR of developing a second primary tumor after neoadjuvant compared with adjuvant RT was 0.64 (95% CI 0.55-0.75; P < 0.0001). Overall survival was independent of radiation sequence (HR 1; P = 0.95). Neoadjuvant RT also resulted in a lower HR for second primary cancer among estrogen receptor-positive patients who underwent mastectomy compared with those who received adjuvant RT (HR 0.48, 95% CI 0.26-0.87; P = 0.0162).
Conclusions: Neoadjuvant RT may significantly improve disease-free survival without reducing overall survival, especially for estrogen receptor-positive patients with early-stage breast cancer. This finding warrants further exploration of potential long-term benefits of neoadjuvant radiotherapy for early-stage breast cancer in a controlled, prospective clinical trial setting, with correlative studies done to identify potential mechanisms of superiority.
Keywords: Disease-free survival; Early-stage breast cancer; Immune response; Overall survival; Postoperative radiotherapy; Preoperative radiotherapy.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–41. doi: 10.1056/NEJMoa022152. - DOI - PubMed
-
- Lerouge D, Touboul E, Lefranc JP, Genestie C, Moureau-Zabotto L, Blondon J. Combined chemotherapy and preoperative irradiation for locally advanced noninflammatory breast cancer: updated results in a series of 120 patients. Int J Radiat Oncol. 2004;59(4):1062–73. doi: 10.1016/j.ijrobp.2003.12.034. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

